From the early days of organic chemistry, the benzoxazine family became noticeable thanks to their interesting core structure. The discovery of 3,4-Dihydro-2H-1,4-Benzoxazin-6-Ol came out of efforts by researchers digging through plant metabolites and synthetic routes that pushed the boundaries of heterocyclic chemistry. This compound, sometimes referred to as DHBO, sprouted from the wider exploration of benzoxazines which, for decades, have offered tools for new polymers, intermediates, and even some bioactive molecules. Chemists working in academic labs through the twentieth century mapped out its initial synthesis, tracing back its reactivity and potential, setting the foundation for what came later in technical development and application.
3,4-Dihydro-2H-1,4-Benzoxazin-6-Ol serves assorted research sectors. With its fused aromatic and heterocyclic ring, the molecule features in pharmacological research, agrichemicals, and polymer chemistry as a versatile synthetic building block. Its structure makes it an interesting candidate not only for academic research but for companies scouting for new material properties or bioactive frameworks. The market gives a range of purity grades; high-purity lots go to analytical or synthetic chemistry labs, while technical grades get routed toward industrial screening work. Over the years, interest has grown within the areas of green synthesis and environmentally friendly materials, building on its chemical stability and modifiable scaffold.
As a crystalline solid, DHBO doesn't demand much special treatment for everyday lab handling. Its melting point hovers within a practical range, allowing for easy manipulation without decomposition during routine reactions. The aromatic system confers stability, while the nitrogen and oxygen take up key reactive spots, enabling further functionalization. Water solubility lands on the moderate side, a pleasant surprise when solubilizing heterocycles can often prove tricky. The molecule absorbs in the UV-visible spectrum, giving analytical chemists an easy handle for quantification or tracking during synthesis. I’ve found this kind of clarity improves daily lab work, letting one avoid headaches over obscure substances that seem to vanish into thin air.
Going through product labels, the IUPAC name appears as 3,4-dihydro-2H-1,4-benzoxazin-6-ol. CAS RN often tags along for traceability, showing up on bottles for regulatory purposes. Commercial batches specify purity, typically pegged at 98% or more, and list out common side products and recommended storage conditions (cool, dark, moisture-free). Shelf life extends out over years if the cap stays tight and the light stays out. Manufacturers include recommended PPE, shipping classes, and identification numbers in compliance with GHS labeling and transport rules. This is more than bureaucratic red tape; it shields handlers from accidental exposure or undefined waste, a lesson hard-learned by anyone who has spent time with outdated chemical stocks.
Preparation of DHBO relies on a key cyclization process, usually starting from ortho-aminophenols and aldehydes or ketones. Many labs follow established condensation reactions, often under mild heating and with acid or base catalysis. One common route mixes 2-aminophenol with formaldehyde, steering the mixture through heated stirring, followed by extraction and crystallization. Yields reach respectable percentages, even for small-scale syntheses. Skilled hands can tune reaction conditions to cut down on byproducts, using straightforward filtration and recrystallization for purification. Scale-up from milligram to kilogram sometimes brings curveballs—changes in solvent or pH can shift the reaction, so consulting the primary literature, or supervisors who have run these before, pays off.
DHBO’s core skeleton takes well to substitutions and functional group transformations. N-alkylation and O-acylation expand its utility, letting chemists tweak solubility, bioactivity, or reactivity for targeted applications. When exposed to electrophilic reagents, both the nitrogen and oxygen supply handles for further attachment. Oxidation and reduction reactions offer entry to different oxidation states of the core, extending access to derivatives that can become dyes, ligands, or pharmaceutical candidates. In practical research, these transformations help create small, targeted libraries for screening in chemical biology or material science. Modifying the structure often turns up compounds with antibacterial or antifungal properties, a bonus for interdisciplinary projects chasing both basic and applied results.
Chemists face enough confusion without ambiguous names, so accuracy matters. Synonyms for 3,4-dihydro-2H-1,4-benzoxazin-6-ol include 2H-1,4-Benzoxazin-6-ol, 3,4-dihydro-, and DHBO. Certain suppliers refer to it as Benzoxazin-6-ol, dihydro-, or simplify to Benzoxazinol. Its CAS number provides the ultimate key for database searches and global tracking. Keeping tabs on synonyms saves time and limits mix-ups, especially when ordering from different suppliers or searching through decades of published literature.
Standard lab safety rules apply, as with any organic compound, but DHBO doesn’t rank among the notorious hazards. Gloves, goggles, and a lab coat keep most risks in check. Direct ingestion or contact with eyes deserves special caution, as mild irritant effects have surfaced in toxicological checks. Proper ventilation, neat workspaces, and labeled waste collection help dodge chemical exposure. SDS sheets published by suppliers provide clear guidelines on first-aid steps, fire hazards, and environmentally safe disposal. Regulatory frameworks such as REACH and OSHA in the EU and US take interest in its handling, ensuring that suppliers spell out transportation and storage rules. Real-world lab experience teaches respect for these protocols—once you see a chemical spill handled poorly, you don't want a rerun.
Research labs dig into DHBO for both fundamental discoveries and applied innovations. In pharmacology, the molecule’s ring system mimics structures found in plant-derived defense compounds, offering templates for new antifungal or antibacterial agents. Agrochemical research taps this compound for potential allelochemical effects—farming ecosystems sometimes benefit from such chemicals embedded in crop rotation systems or as leads for selective herbicides. Polymer developers eye benzoxazine monomers for next-generation materials, including high-heat, fire-retardant plastics. DHBO fits right in, giving engineers a way to tune performance without reaching for more hazardous inputs. Small-scale medicinal chemistry efforts often look at DHBO as a way to scaffold bioisosteric replacements, seeking breakthrough properties for new drugs or diagnostics.
Academic groups publish new findings every year, tweaking DHBO’s structure for better biological action or polymer performance. Computational chemistry has made headway in predicting the bioactivity and reactivity of new DHBO derivatives, saving time and resources in the lab. High-throughput screening and structure-activity relationship studies contribute to a growing bank of knowledge about its potential, especially as a lead candidate for antifungal and anti-infective research. In industry, pilot batches and product samples feed into development pipelines for greener, safer chemicals. International collaborations widen the circle, as researchers share best practices and novel approaches, making progress faster than any solo operator could manage.
Toxicology studies so far rank DHBO as low to moderate in acute toxicity, with limited absorption through the skin and quick elimination pathways in animal trials. Chronic exposure data remains thinner on the ground, so long-term occupational studies and careful monitoring still matter. In plants and aquatic organisms, runoff and breakdown products need evaluation before any push toward widespread environmental release. Emerging data tracks tissue effects, cellular metabolism, and mutagenicity, giving regulators and safety boards enough to draft preliminary guidelines. A responsible pace helps keep research on track without sacrificing safety, reflecting the lived experience of those who have handled unknowns in the past.
Interest in benzoxazine chemistry continues to rise as technology looks for non-toxic, renewable, and high-performance materials. With material science, green chemistry, and biotech investing more heavily in heterocycles, DHBO gets a solid spot in future plans. Research may unlock selective crop protection agents or new polymers fit for extreme environments. If regulatory studies confirm safety and environmental compatibility, agricultural and pharmaceutical rollouts will accelerate. Investment in R&D often hinges on this kind of dual potential—tradition-backed compounds with modern adaptability. As someone who has watched old research chemicals find new life again and again, DHBO’s best days may still lie ahead.
The name sounds like something straight out of a chemistry textbook, but 3,4-Dihydro-2H-1,4-Benzoxazin-6-ol plays a bigger role in the everyday world than most people realize. This compound shows up in places where you’d least expect it: fields of corn, wheat, and rye. It’s not a laboratory invention meant just for scientists; it’s nature’s own defense tool, developed and used by certain plants.
I’ve seen farmers deal with pests, weeds, and plant diseases year in and year out. Struggling with fewer chemical options, they turn to nature for support. Some grains make this benzoxazinone compound to help protect themselves. It works as a built-in shield that fends off insects and slows down certain fungi. That matters a lot for folks who want to keep food production clean and cut back on pesticide use. Research from agronomy journals highlights how this molecule’s role in natural pest resistance reduces the pressure to spray chemicals — that’s money saved and less runoff into waterways.
Nature rarely hands out just one benefit. 3,4-Dihydro-2H-1,4-Benzoxazin-6-ol, often shortened to DIBOA, doesn’t just scare off bugs; evidence from field trials shows it also controls some weeds. Roots of crops release it into the soil. Rival plants that try to sprout nearby find it tough to survive. This natural weed suppression keeps fields clearer, especially in conservation farming where tillage is kept low. Since soil disturbances are kept low, weed seeds don’t get buried or destroyed — the natural allelopathy makes a big difference.
These days, folks worry about food security and the side effects of agricultural chemicals. I’ve heard concerns about pesticide residues and ecological impacts from many in the food movement. DIBOA and similar plant-made compounds offer another path. By breeding crops for higher production of this natural chemical, producers strengthen the plant’s own immune systems. Peer-reviewed studies point out fewer fungal infections and better crop vigor. That means a bigger, more stable harvest — something everyone in the community can get behind.
The story doesn’t stop at plant defense. Some lab studies have explored whether benzoxazinone derivatives could fight bacteria or even hold clues to designing new medicines. That research isn’t ready for doctors’ offices yet, but it sparks hope. Language from medicinal chemistry journals hints at mild antibacterial properties, though human trials remain far off. Even so, this reveals just how much untapped value sits inside wild chemistry first built by plants.
Challenges still exist. Not every grain produces high amounts, and environmental factors influence how much is made. Some weeds and pests develop ways to shrug off these defenses over time. Investment in breeding programs, genetic research, and close partnerships between farmers and scientists will set the stage for bigger gains. If I could wave a magic wand, I’d bring more attention to these plant-made protectors — because smarter agriculture starts with listening to what plants do best.
Folks everywhere want safer food and cleaner ecosystems. Plant chemistries like DIBOA show the power of leaning into natural strengths. Building up these tools could help cut down on synthetic pesticides, support more sustainable farming, and maybe even inspire new medicines in the long run.
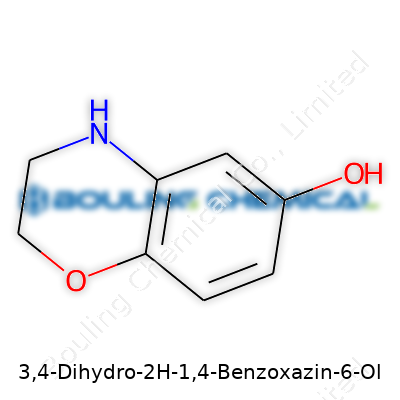
The name "3,4-Dihydro-2H-1,4-Benzoxazin-6-ol" sounds complicated at face value, but taking it apart gives us a look at a distinctive bicyclic structure. The backbone starts with a benzene ring fused to an oxazine ring. In chemistry labs, this kind of molecule stands out for its clever arrangement of atoms; there’s a nitrogen and an oxygen sharing the second ring, with a fresh hydroxyl group dangling off the sixth position. Put simply, this molecule combines both aromatic and heterocyclic worlds.
The structure doesn’t only catch the eye. At the atomic level, the placement of that hydroxyl group and the configuration of the benzoxazine make it uniquely reactive. Speaking from experience in chemistry classes, lab experiments involving benzoxazine derivatives often show just how much a small tweak changes everything. Small changes in a molecular skeleton can turn an inert compound into something remarkably active—sometimes for pharmaceuticals, other times for plant biology.
Nature offers examples. Maize plants produce benzoxazinoids—relatives of our molecule—when facing pest attacks. The six-position hydroxyl supports not only solubility but reactivity, helping the plant defend against invaders. It’s a lesson in how a simple arrangement can deliver large-scale effects in living systems.
In the hands of chemists, 3,4-Dihydro-2H-1,4-Benzoxazin-6-ol becomes more than a theoretical structure. Some research investigates how derivatives act as bioactive agents. In pest control, certain substitutions on the benzoxazine core enhance activity without introducing toxicity to non-target species. Sustainable agriculture depends on solutions like these, where understanding structure can lead to tools that protect crops and the environment.
Another area relates to pharmaceuticals. The benzoxazine scaffold gets explored as the backbone for new drug candidates. That arrangement of arene and heterocyclic elements lets chemists attach groups to the ring, tuning biological activity and aiming for better targets—safer antitumor agents, for example, or anti-inflammatory compounds. Medicinal chemistry never moves far from the principle: what you put where on a molecule’s frame makes a difference.
Understanding chemical structure demands a mindset of curiosity. Textbooks give the rules, but real insight comes from connecting atomic placement to real-world results. I remember struggling through skeletal formulas in college, trying to see why an extra oxygen here or a nitrogen there mattered at all. Eventually, I realized that those subtleties formed the basis for everything from material durability to biological effectiveness.
For those tackling global challenges—crop loss to pests, resistance to medicines—this mindset matters. Research that explores structure-activity relationships paves the way forward. Open access to chemical data, supporting collaboration across universities and disciplines, helps turn knowledge about structure into practical results. Efforts to share findings help move discoveries out of journals and into the fields where food grows or the clinics where patients are treated.
In the end, 3,4-Dihydro-2H-1,4-Benzoxazin-6-ol stands as a case study in the power of looking closely at structure. Chemists, biologists, and engineers can’t afford to overlook how those connections among atoms ripple through whole systems. Solutions don’t always look dramatic at first glance, but they often depend on the details hidden in a molecular shape.
3,4-Dihydro-2H-1,4-Benzoxazin-6-ol doesn’t roll off the tongue. In labs and in the chemical industry, it shows up enough to draw attention. People sometimes ask if it’s safe to work with, and that’s worth talking about as simply as possible. I’ve handled a lot of chemicals with strange names in my time. Care always matters, no matter how harmless a compound might seem at first glance.
Research tells us that benzoxazinone compounds, in general, can interact with living cells. This one—the 3,4-dihydro version—pops up in studies about plant defense molecules and sometimes in synthetic chemistry projects. It hasn’t been singled out as especially dangerous. There’s limited information, though, and that means people should avoid guessing. Even compounds without a row of hazard symbols on the lab shelf can cause trouble if skin absorbs them or if you breathe them in.
Most exposure worries fall into three big categories: skin contact, eye contact, and inhalation. If powdered or dissolved benzoxazinols get on your skin, you can’t always count on an immediate burning sensation. Some compounds sneak in under the radar and trigger reactions over hours or days. A number of benzoxazine-type molecules carry risks of inflammation, irritation, or even more lingering health effects if mishandled for months on end.
Breathing dust or fumes always brings risks, even if information is patchy. Not every chemical with an unremarkable safety sheet is truly harmless, and everyone has heard stories of “mild” solvents leading to headaches or skin problems. Personal experience tells me running water, gloves, and goggles are more than just overkill—they stop problems before they start. My old mentor’s rule still stands: don’t trust your guesswork, trust the gear.
Stored properly, most benzoxazine compounds last, but they can change if left in sunlight or open to the air. Without detailed breakdown studies, nobody should treat the breakdown products as safe either. Small lab fires have started from compounds with similar structures reacting with oxidizers, based on accident reports shared in university lab safety meetings.
Disposal adds another layer of concern. Waterways can turn into chemical highways, and municipalities rarely handle obscure organic chemicals with perfect precision. Even a few grams washed down a drain add up across cities and years. Anyone handling these compounds ought to rely on hazardous waste pickups instead of shortcuts.
Every time I prep a new compound, a checklist keeps me honest: gloves, lab coat, splash-proof goggles, fume hood. Washing up, even after short use, creates habits that pay off. Accidents feel unlikely until they happen. In spaces without proper ventilation or if your workplace skips on safety supplies, stop and make noise about it. Rushed chemistry leads to real pain, not just splashed shirts.
Training matters just as much. Reading material safety data sheets saves more skin than any homegrown strategy. Universities sometimes treat chemical safety as an afterthought, but the graduates who stick around the longest ask questions, and they learn from the old scars on their mentors’ hands.
Some will say the risks sound inflated, especially with chemicals that look like mere research curiosities. I’ve seen grad students regret casualness and postdocs leave research after routine exposures caused rashes. Unfamiliar names invite mistakes. Safety routines protect not just the handler, but everyone in the workspace, and even people outside it who never knew what got poured down that sink. No reputation in chemistry or biotech grows from playing it loose with unknowns.
3,4-Dihydro-2H-1,4-Benzoxazin-6-ol may sound like just another entry in a long chemistry textbook, but careful handling really makes a difference. Experience around lab stocks has shown me that tiny slips—like leaving a sample out on the bench—can lead to headaches, lost money, and even risks to health. Given how many research and industrial settings rely on specialty compounds, understanding how to store this compound isn’t just about ticking off a safety checklist—it protects both people and research budgets.
Chemists know that temperature often determines how long a compound stays stable. For 3,4-Dihydro-2H-1,4-Benzoxazin-6-ol, room temperature sometimes feels safe enough, but this not only shortens its lifetime. Chemical breakdown—hydrolysis or oxidation—becomes a real risk. Refrigeration, around 2-8°C, improves shelf life and cuts down on unwanted reactions. It may seem like an extra step, but pulling out the cold storage after shopping for expensive chemicals is standard practice across reliable labs.
Light introduces another wrinkle. I’ve seen more than a few bottles on open shelves turn a strange shade—or worse, lose their punch—thanks to excess sunlight. Shielding the compound in amber glass or another opaque container goes a long way to slow down photodegradation. Photochemistry sneaks up quickly, especially in labs with strong overhead lighting.
Oxygen exposure presents its own hassles. Drawing samples, leaving caps loose, or handling in a damp environment encourages slow oxidation or moisture uptake. Silica gel and tight-sealing vials keep things on track. Chemical suppliers often print “store under inert atmosphere” on the label, and based on real-world practice, it pays to listen. When opening a new bottle, I’ve always kept the exposure brief, then opened the glovebox or flooded the space with a gentle flow of nitrogen.
No one wants to dig through a freezer only to find a pile of mysterious vials, half-faded labels, or sticky residue. Taking the time to write clear dates and batch numbers, then logging everything into a notebook or electronic system, matters more than it might seem. When results go sideways, or a project needs to confirm a batch’s identity, these basic records save hours. Skipping this step leads to wasted experiments and, potentially, publication retractions.
I’ve made a habit of regular checks. Every few weeks, cracking open the fridge, scanning for leaks, off-colors, or weird smells keeps surprises at bay. If anything seems off, segregating the sample or reaching out to a chemical waste officer feels tedious but avoids bigger problems later.
Building good habits starts on day one. Education, reminders, and walk-throughs from senior lab personnel help. Investing in the right storage supplies—desiccators, proper refrigerators, and easy-to-read labeling gear—always proves its worth in saved chemicals and peace of mind. Teams where everyone feels responsible for safe storage tend to have fewer ruined samples and safer working conditions.
In short, taking storage seriously benefits everyone—those who handle chemicals, the researchers banking on reliable data, and the broader community depending on safe labs. It’s a small price for trust and progress.
College research left a lasting impression on me. Chemicals like 3,4-Dihydro-2H-1,4-Benzoxazin-6-ol never pop up at the local hardware store, and there’s a reason behind that. They matter more for their use in the lab than on any shelf. This compound brings utility in plant science and pharmaceutical research. With potential as a biochemical tool or in synthesis, it has stirred up more than one patent filing. That sounds impressive, but it’s not something you can just order from an online marketplace with free shipping.
Checking the supplier’s credentials goes a long way in chemical procurement. Many labs, myself included, lean on trusted chemical suppliers like Sigma-Aldrich, TCI, Alfa Aesar, and ChemFaces. These names offer more than a label — they provide purity reports, storage recommendations, and regulatory documentation. Buying from a no-name distributor can endanger both the project and everyone involved. One research group lost months because their “bargain” supplier sent low-purity stock, and their gene expression experiments fell apart. It hurt their credibility and wasted their grant.
Regulation puts a clear barrier on the purchase of many chemical substances. 3,4-Dihydro-2H-1,4-Benzoxazin-6-ol sits in a gray zone for some countries, triggering extra paperwork, permits, and chemical registries. In the US, major chemical companies demand institutional accounts for controlled or uncommon compounds. Nobody walks in and pays cash. Some places require professional licenses or end-use statements, especially if the chemical straddles categories like research use only or precursor material. Oversight may be strict, but there’s a reason: accidental exposures and illegal diversion both create real harm, so jumping through these hoops is part of the job.
A memory sticks with me from grad school — a minor chemical spill from a mislabeled container resulted in evacuation and two anxious days of waiting for toxicology reports. The lesson: every chemical purchase should start with a plan about its storage, use, and disposal. Chemicals like 3,4-Dihydro-2H-1,4-Benzoxazin-6-ol should be treated as a managed asset, not a consumable. Emergency protocols, SDS access, and compliance with institutional safety rules can’t be skipped. The people sharing your lab love science as much as you do — and deserve to come home safe every night.
Labs should keep lists of approved vendors, encourage vetting of new sources, and regularly update compliance training for all users. Researchers can use online peer forums or university chemical safety offices for trade recommendations and blacklist alerts. Some organizations team up with bulk purchasers to cut costs while keeping oversight high. Routine audits, project-based inventory management, and transparent procurement all help keep research moving forward without looking over your shoulder.
Once you appreciate the stakes, shortcutting the process feels reckless. The real solution lies in responsibility: knowing where the chemical comes from, understanding its risks and benefits, and respecting the regulatory landscape. Buying a powerful research tool should always require diligence, collaboration, and respect for the spirit of science.
| Names | |
| Preferred IUPAC name | 3,4-dihydro-2H-1,4-benzoxazin-6-ol |
| Other names |
6-Hydroxy-3,4-dihydro-2H-1,4-benzoxazine 6-Hydroxybenzoxazine DIBOA Benzoxazin-6-ol, 3,4-dihydro-2H- 2H-1,4-Benzoxazin-6-ol, 3,4-dihydro- |
| Pronunciation | /ˌtraɪ.haɪˈdrɒks.i.bɛnˌzəʊˈæz.ɪnˈɒl/ |
| Identifiers | |
| CAS Number | [23616-01-1] |
| Beilstein Reference | 130207 |
| ChEBI | CHEBI:28544 |
| ChEMBL | CHEMBL18933 |
| ChemSpider | 110098 |
| DrugBank | DB08346 |
| ECHA InfoCard | ECHA InfoCard: 100.044.076 |
| EC Number | 1.14.13.140 |
| Gmelin Reference | 1641515 |
| KEGG | C05669 |
| MeSH | D015005 |
| PubChem CID | 151920 |
| RTECS number | KF1530000 |
| UNII | A136C4L9ZS |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID60151187 |
| Properties | |
| Chemical formula | C8H9NO2 |
| Molar mass | 151.16 g/mol |
| Appearance | White to light yellow crystalline powder |
| Odor | Odorless |
| Density | 1.33 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 0.3 |
| Vapor pressure | 4.5E-4 mmHg at 25 °C |
| Acidity (pKa) | 7.87 |
| Basicity (pKb) | 5.13 |
| Magnetic susceptibility (χ) | -0.64 x 10^-6 cm^3/mol |
| Refractive index (nD) | 1.672 |
| Viscosity | 760.3±47.5 mg/mL |
| Dipole moment | 2.58 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 322.8 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | -201.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –3714 kJ/mol |
| Pharmacology | |
| ATC code | N06AX17 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS05,GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P280-P305+P351+P338-P337+P313 |
| Flash point | 111.5 °C |
| Autoignition temperature | 400 °C |
| LD50 (median dose) | LD50 (median dose): Rat oral 670 mg/kg |
| NIOSH | RN 3621-82-1 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100 mg |
| Related compounds | |
| Related compounds |
2,3-Dihydro-1,4-benzoxazine 2,3-Dihydro-1,4-benzoxazin-6-ol 1,4-Benzoxazin-3-one 4H-1,4-Benzoxazin-3-ol 4H-1,4-Benzoxazin-3-one |