Interest in 1,2-benzisothiazoles dates back to mid-20th century, when medicinal chemists moved beyond the usual amine and benzene tinkering and started exploring heterocycles. By the 1970s, a handful of small labs across Europe and North America were investigating ways to connect piperazine to isothiazole backbones, looking for new central nervous system agents on the heels of chlorpromazine’s success. Over many years, libraries of similar molecules grew. I remember reading old notebook scans from university archives—chemists were optimizing yields with whatever glassware worked, mixing up tiny batches, jotting down melting points, exploring over 30-40 derivatives of simple structures, and always searching for something that’d make a difference. 3-(1-Piperazinyl)-1,2-Benzisothiazole showed up as a candidate scaffold right around the era when both pharmaceutical research and patent activity exploded, particularly in the late 1980s, as antipsychotic drug development ramped up.
Put simply, this is a fused sulfonyl-heterocycle with a piperazine ring at the three-position. Researchers regularly refer to it as a privileged structure because it’s a springboard for all sorts of analogues used in medicine. The molecule sits in a sweet spot: stable enough for scale-up, reactive enough for modifications, small enough to enter the brain efficiently, and “drug-like” because it behaves predictably during formulation. It rarely shows up as a product on a drugstore shelf, but its backbone forms the foundation for prominent treatments, especially in psychiatry.
I recall the first time I handled samples—fine pale powders, sometimes off-white to light tan, with a mild musty scent (not unlike other sulfur-containing chemicals). The compound handles moisture okay; it won’t degrade fast if capped tight, although strong light shouldn’t hit it too long. Water solubility trends low, but polar solvents or a touch of acid (since the piperazine can pick up protons) gets it moving. Its melting point often sits between 160–175°C. The ring system itself brings some rigidity; side-chains or N-alkyl substitutions bump the melting point a little. Analytical chemists find it easy to follow by UV or LC-MS methods, thanks to the benzene and isothiazole rings absorbing strongly.
Suppliers label this compound by CAS number—either 87691-37-2 (unsubstituted) or with various registry numbers for its salts. Quality specs demand over 98% purity for any use closer to R&D or pharma, and less stringent lots for chemical intermediates. Usually, data sheets cover melting range, HPLC chromatogram, and a mass spec trace. Sample vials state net weight, moisture limits, and batch code.
At the bench, many labs use a cyclization route. They couple an o-aminothiophenol with the right piperazinyl acetyl chloride or corresponding acid, controlling stoichiometry, then heat, sometimes adding a mild oxidant like ferric chloride or iodine in a solvent like dichloromethane. Other variations use sodium nitrite under mild acidification, or catalysis with copper(II) salts. Purification means repeated extractions—organic washes, aqueous workups, and a final crystallization from ethanol or ether to get a pure solid. The biggest issue centers on over-oxidation, which can chew up the ring if the operator isn’t vigilant about temperatures and pH.
Chemists see this structure as a kind of workhorse. The piperazine ring welcomes alkylation, acylation, or even deuterium labeling. The benzisothiazole core opens the door for halogenations, nitrosations, or Suzuki couplings, letting researchers bolt on anything from small hydrophilic groups to massive aromatic arms. In pharma settings, small changes to the piperazine shift the central nervous system activity drastically—swapping one methyl for another halogen can often make or break a clinical candidate. The chemistry stays relatively robust: most modifications take place on N-atoms, or with organometallic catalysts that tolerate the isothiazole backbone.
In research circles and suppliers’ lists, this molecule goes by several names. Common entries include “1,2-Benzisothiazol-3-ylpiperazine,” “1-Piperazinyl-benzisothiazole,” or the short “BZP-pip.” It pops up under chemical supply houses’ house brands, typically paired with suffixes for hydrochloride or hydrobromide salts. Regulatory documents sometimes tack on registry numbers or FDA IND codes if it’s made it to advanced studies.
No one takes safety shortcuts lingering around benzisothiazoles, especially under the fume hood. Chronic exposure research has flagged possible skin and respiratory irritation. Chronic rodent studies aren’t pretty, reporting liver enzyme elevations and rare cardiovascular signals. Gloves, glasses, and a working hood count as the bare minimum, with any spills cleaned by trained hands. Labs observe strict limits for airborne concentration, and most companies require dedicated logs tracking quantity and usage by personnel, especially since derivatives are scheduled in several countries due to potential psychoactive effects.
This molecule didn’t grab headlines as a pure compound, but its analogues sit at the core of antipsychotic and antihistamine drug patents. Ziprasidone and related molecules, used for mental health and depression, depend on this structure. In chemical biology, its derivatives act as probes for dopamine and serotonin receptors. Out in agricultural chemistry, some teams have tweaked the ring to fight fungal growth, but the main action stays in the human health space. Medicinal chemists use it for its “privileged” properties, meaning it consistently generates new leads for CNS targets without wild, unpredictable side effect profiles.
Drug hunters return to the isothiazole-piperazine template year after year, blending computational chemistry with old-fashioned wet lab synthesis. The molecule’s robust synthetic accessibility, tolerance for late-stage functionalization, and CNS-penetrant qualities keep it in play at both universities and industry R&D parks. A lot of time, I’ve watched teams run dense screens tweaking ring substituents, looking for next-gen antipsychotics that dodge metabolic pitfalls of current drugs. Machine learning tools continue to scan isothiazole libraries, mapping out new candidates for overlooked or emerging CNS targets. Major pharma’s patent filings keep citing this backbone, showing it hasn’t faded into chemical obscurity.
No compound escapes scrutiny these days, and 3-(1-Piperazinyl)-1,2-Benzisothiazole has weathered more toxicology screening than most chemical scaffolds. Early mouse studies mapped out hepatic metabolism and flagged a few rare toxicities—probably due more to certain substitutions than the core itself. Researchers keep a close eye for liver and kidney markers, and some aryl derivatives at high doses caused convulsions or weight loss in animal models. Regulators demand carcinogenicity and mutagenicity runs, but epidemiological links haven’t shown up in patients using the drug derivatives. Regular workers get baseline bloodwork if they handle it often, just as an extra precaution.
It’s easy to think research will move on, but the industry keeps finding fresh approaches with the benzisothiazole-piperazine scaffold. Companies aim for new antidepressants, cognitive enhancers, and treatments for neuroinflammation by playing with substituent chemistry. In synthetic chemistry, green methods with lower waste streams and improved atom efficiency look promising to streamline both scale-up and environmental impact. Academic groups push the boundaries with more targeted labeling for receptor mapping, pushing the scaffold into chemical biology as much as clinical science. With computational models improving and safety standards tightening, the lifespan of this structure looks far from over, carrying new hope for neurological research and patient care.
Pharmaceutical labs buzz about molecules with long names and complex rings, but 3-(1-Piperazinyl)-1,2-Benzisothiazole wins interest for specific reasons. It gives medicinal chemists a reliable starting point when building drugs that affect mood and cognition. I’ve followed many research updates, and this structure pops up again and again, especially in the search for new antipsychotics. The benzisothiazole core isn’t just a fancy ring—it becomes the “anchor” that holds together ideas for new treatments in mental health.
Drug developers lean on this molecule when creating medicines for disorders like schizophrenia. One of the most discussed examples is ziprasidone, which is used to manage symptoms of psychosis and bipolar disorder. Before medicines like these, choices felt limited. Side effects dragged on, and doctors struggled to find the right balance between effectiveness and problems like drowsiness or agitation. So the piperazinyl-benzisothiazole skeleton gave the field a jolt of new hope.
The molecule acts as a “handle” that scientists use to grab onto dopamine and serotonin targets in the brain. Think of it as a wrench sized just right for a stubborn bolt—engineers can swap out the grip or change the length, and suddenly they have a tool that fits exactly where it needs to. This flexibility means chemists can experiment with small changes and keep discovering new treatments.
In the past decade, research journals filled up with studies about newer analogs and derivatives. People want drugs that work for more patients with fewer side effects. This structure continues to attract attention because chemists believe there’s more to squeeze out of it. By introducing different “side arms” to this molecular frame, teams hope to create treatments for anxiety, depression, and even conditions without solid options today.
Patent databases carry plenty of entries mentioning this backbone. That’s a sign investors see something reliable in its chemistry. Having worked alongside researchers focused on brain health, I see how hard they chase molecules that cross the blood-brain barrier efficiently but don’t sit in the body forever. 3-(1-Piperazinyl)-1,2-Benzisothiazole gives them just that edge.
Setting up production lines for specialized chemicals drains resources. The synthesis route for this compound isn’t trivial—it asks for pure raw materials, controlled reactions, and serious quality checks. I’ve walked through chemical plants where minor mistakes in timing or temperature send entire batches to the scrap heap. This precision partly explains why medicines based on this structure often come with a hefty price tag.
For the next wave of drug innovation, accessibility matters as much as science. People crave mental health solutions that work across different backgrounds. Researchers could reach out more, join forces with academic groups or government labs, and share insight instead of fencing it in patents. That approach leads to new ideas, a broader pipeline, and—hopefully—healthier lives for those who need it most.
So, this molecule does more than sit on a shelf in a chemistry catalog. It helps shape the future of mental health care, giving scientists and doctors a stronger starting line to run the next lap in drug discovery.
Anyone who steps into the world of chemical research learns pretty quickly that purity isn’t just a number on a certificate. It’s the difference between a reaction that works and one that blows up your week, budget, or both. Take 3-(1-Piperazinyl)-1,2-Benzisothiazole for example. Chemists put this compound to use when synthesizing antipsychotic agents and other pharmaceuticals. The target properties of a new drug rest on having the right starting material, so you don’t want surprises lurking in the bottle.
Labs and industry ask for purity levels of at least 98%–sometimes even 99% or more if they’re mapping out a clinical route. This is usually measured by high-performance liquid chromatography (HPLC). When I’m checking a spec sheet, my eyes scan to see if they call out the main remaining 1–2% — you want to know which minor impurities you face, not just the count. The chemical world loves details, not guesswork.
Nobody wants their project to fall apart because the bottle had too much moisture or leftover solvent. For this compound, you’re looking for a white to pale yellow crystalline powder with a melting point around 120°C to 124°C. That window says the bulk matches the molecular structure you expect. As for trace metals, labs want less than 10 ppm of heavy metals. Water content goes under the microscope, rarely above 0.5%, since that much can trigger side reactions or impact crystallization. Lost time chasing a batch that won't dry comes from shortcuts on drying or careless storage.
Packing irregularities sometimes show up as the compound slowly soaks up air moisture, turning sticky or clumpy. Analytical tricks like Karl Fischer titration let you flag water before it goes further. Chemical identity isn’t just carbon and nitrogen on a technical sheet — real confirmation calls for NMR, IR, or mass spectrometry. I once dealt with a batch that looked normal, but trace NMR signals tipped us off to leftover piperazine that nearly derailed a series of small-scale screens. Quality control is about asking questions, not blindly trusting a label or supplier’s promises.
It’s common to see this compound distributed through both large catalog suppliers and smaller, lesser-known traders. Some sources cut corners, skipping robust testing or transparency. This shows up as mystery peaks in lab analyses or, worse, unsafe work conditions during use. A project only moves forward when chemists and buyers put trust in raw specs, but they also check certificates. Story after story circulates about rushed synthesis prepping the route for failure further down. Bad or misidentified material blocks drug discovery outright.
Introducing validated, public-facing standards for this and similar compounds takes out a lot of guesswork. Third-party testing, open communication between manufacturers and users, and feedback loops where labs review their purchases can shut down a bad batch before it triggers problems further along the pipeline. More people in the loop means fewer costly mistakes and more reproducible science. The chemical world runs on small numbers, but big consequences if someone ignores the specs.
A chemical like 3-(1-Piperazinyl)-1,2-Benzisothiazole carries a name that might trip up your tongue, but the story around its safe use comes down to a few commonsense rules. You step into any lab, and you see bottles and vials labeled with names most folks haven’t heard before. Behind each one sits the possibility for real harm if handled the wrong way. I learned early, working nights in a small pharma lab, that familiarity with procedures saves more fingers than most people realize.
Chemicals thrive in stable environments—swinging temperatures or high humidity spell trouble. I always set aside dry, cool spots for sensitive vials. For 3-(1-Piperazinyl)-1,2-Benzisothiazole, skipping a climate-controlled shelf might seem harmless. But left in a warm room or near sunlight, any organic compound risks breaking down or producing vapors. A locked cabinet means nobody grabs the bottle by mistake. Chemical suppliers recommend temperatures below 25°C and containers that keep moisture and air away. If you wouldn’t store food there, don’t stash your chemicals there either.
No matter how routine a task feels, skipping the step of checking a label leads to disaster. Once, I caught a co-worker about to pour something sticky and colorless—almost clear ethanol—into the disposal sink, marked only as “solvent.” Turns out, it was not ethanol at all. Whether in a high-end research building or a school teaching lab, you can’t rely on memory alone. Clear, accurate labels, with dates and hazardous symbols, help everyone in the room know exactly what they’re picking up.
You won’t catch me in a lab without gloves, goggles, and a decent coat, not anymore. Maybe in the early days, I thought I could handle a quick transfer with bare hands, but burns and rashes changed my habits for good. This compound, like many, can irritate skin and eyes. Extra caution with splash protection, lined gloves, and proper ventilation saves a trip to the emergency room. If you can, prep and use chemicals inside a fume hood—not just for your own lungs, but for everyone sharing the air.
Disposing of leftovers or spills takes a moment of thought, not a quick dump down the drain. I’ve seen drains clog and fumes rise from folks treating every liquid as the same. Segregated waste bottles, marked for organic or halogenated solvents, reduce accidents. Many compounds react unexpectedly with cleaners or water, so segregating your bottles—using checklists or color-coding—saves money and trouble. Your campus or workplace will offer disposal instructions, and following them to the letter means you avoid future headaches.
I never trust my memory from last year’s safety talk. Regular updates, hands-on practice with spill kits, and reviewing new protocols help everyone stay sharp. In my time training undergraduates, nothing replaced the quick, in-person drills on glove changing or emergency showers. Resources from chemical suppliers and university safety offices offer up-to-date data, including Material Safety Data Sheets (MSDS). Keep copies where anyone can grab them, and make time to talk openly about mistakes or near misses.
Chemicals with complex names often come with complex hazards. Habits matter more than rules written on the wall. Protect yourself, keep storage smart, and treat every container like it demands respect. From locked cabinets to constant training, it’s the details—done right every day—that make accidents the rare exception instead of the norm.
I’ve spent years working with specialty chemicals in research and sourcing roles. Every time a compound like 3-(1-Piperazinyl)-1,2-Benzisothiazole crosses my desk, the same set of questions pop up: Can we get enough of it? Who actually produces it in commercial quantities? Costs, lead times, regulations — that’s what shapes access, not just a catalog listing.
Anyone in pharma or advanced materials will spot that molecular scaffold right away. Core to antipsychotic drug research, sometimes branching into specialty polymers, its structure isn’t just a curiosity. It’s the gateway molecule for a niche set of downstream products. But niche means volume is tricky.
Here’s what happens. Research-scale needs — a few grams or maybe tens of grams — get met by lab suppliers. Once demand jumps into the kilograms, the real hunt starts. Chinese and Indian factories sometimes list “bulk” quantities, but after calling around, you find out that real, repeated production isn’t guaranteed. More than once, “in stock” turns into two months’ wait, with the fine print showing “custom synthesis only.”
Regulators have a close eye on compounds with psychoactive potential or chemical similarities to controlled drugs. 3-(1-Piperazinyl)-1,2-Benzisothiazole sometimes lands in a gray area, not blacklisted, but not exactly free to ship anywhere, either. Anyone buying in bulk for commercial use must face shipping restrictions and compliance hoops set by REACH, TSCA, or similar agencies. Documentation piles up, and logistics costs rise, sometimes unexpectedly.
Chemical suppliers quote prices by the kilo, but there’s a catch: kilo prices leap when procurement jumps from R&D to pilot production. Single digits per gram can multiply manyfold at larger scales because these aren’t ton-scale commodity chemicals. Supply chains already feel it when one plant goes offline, or a precursor becomes hard to source. In my experience, companies sometimes split orders between vendors just to hedge risk, and buyers stick to contracts year-to-year. Spot buying is a gamble.
Those seeking bulk access don’t just shop around, they build relationships. If a company expects multi-kilo flows, the smartest move is early talk with producers about forecasts and batch scheduling. Labs often pay to reserve future batches because waiting in line means lost months on drug programs or materials production. Some move toward local toll manufacturers for more supply control, but then must share core intellectual property or custom process details. Trust runs both ways.
The global pandemic taught us that specialty chemical supply can unravel fast. Stockpiling is costly for low-turnover compounds, but keeping a strategic safety buffer doesn’t seem so wasteful anymore. Buyers and producers benefit from better communication about actual near-term needs. On the regulatory front, those pushing for more transparent export controls and harmonized rules take a load off capable suppliers.
Bulk quantities of 3-(1-Piperazinyl)-1,2-Benzisothiazole sit at a crossroads between science and business realities. Sourcing takes more than a catalog search and wire transfer. For many, the path to reliable access runs through long-term negotiation, smart contracts, and staying ahead of the next policy twist — all steps I’ve come to respect after years on the phone with factories around the world.
For anyone who’s dealt with chemical purchasing, “lead time” isn’t some vague phrase tossed around in emails. It shapes everything from your project’s calendar to your boss’s mood, right down to the science—a late delivery brings experiments to a halt and draws complaints from every direction. These problems seem small until you’re stuck with idle staff or the lab’s down for days because a shipment sat at a border.
Based on what I’ve seen in chemical sourcing, lead time on 3-(1-Piperazinyl)-1,2-Benzisothiazole runs anywhere from a few days up to several weeks. The clock starts not at the time of order submission, but often only after payment lands and paperwork lines up. Domestic suppliers finish things faster if they hold stock in-country, usually shipping out within three to five business days. If the supplier needs to synthesize the compound, a two- to four-week wait rolls into the mix.
China and India keep a tight grip on production of specialty chemicals. Ordering direct from these regions tends to take longer. I’ve seen international shipments run about two to four weeks just to leave the port—customs checks on controlled substances and proper handling of documents keep trucks waiting. This isn’t academic; I’ve watched shipments stuck for a week simply because one little stamp was missing.
Air freight dominates when a timeline grows short. Given the cost, air works for lab-scale purchases and time-sensitive runs. The main risk with air comes from paperwork: shipping chemicals demands the right safety certificates, including SDS, certificates of analysis, and sometimes import licenses. One missing form, and cargo hangs in limbo at an airport warehouse, adding surprise storage fees and hours on the phone for everyone involved.
Bulk buyers chasing costs often turn to sea freight. Cost per kilo drops in larger lots, but ocean shipping crawls along in contrast. Four to eight weeks is the usual pace, longer if the receiving port runs behind. Anyone who’s sweated through a delayed ocean delivery will know what it feels like—no one wants to explain to a sponsor why samples for the next batch won’t show up for another month.
Some companies use their own logistics channels for greater predictability. Dedicated forwarders build in customs handling and clearances as part of their pitch, which appeals to buyers burned by prior delays. We learned the hard way after losing a container to customs for three weeks: always push for up-to-date tracking and pick a forwarder with chemical experience.
The type of shipping—hazardous or regular—depends on the formulation. Since 3-(1-Piperazinyl)-1,2-Benzisothiazole often falls under “hazardous” classifications depending on country, hazmat handling inflates both lead time and freight costs. Regular follow-ups with your supplier and broker pay off here; they’ll spot hold-ups before they snowball into bigger problems.
None of these issues mean delays are inevitable. Good communication prunes hours off sourcing. Getting MSDS and import permits settled before ordering knocks whole days off the clock. In my work, double-checking import codes and talking with customs officials before placing international orders turned out to be the ticket for smooth delivery. It’s not fancy: it’s calling, emailing, getting things in writing. Chemistry isn’t magic, nor is logistics. Once you treat purchasing like a process with plenty of variables—just like a reaction—risk drops away and shipments show up closer to when they’re needed, keeping researchers happy and projects moving forward.
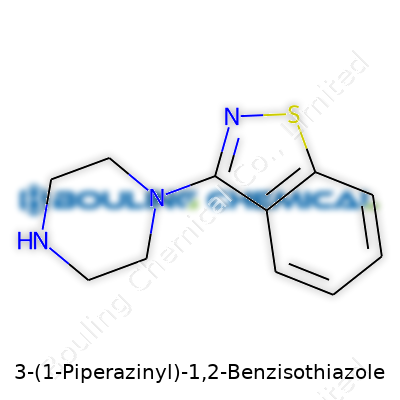
| Names | |
| Preferred IUPAC name | 3-(Piperazin-1-yl)-1,2-benzothiazole |
| Other names |
1,2-Benzisothiazol-3-ylpiperazine 1-Piperazinyl-3-benzisothiazole 1,2-Benzisothiazole, 3-(1-piperazinyl)- 3-(Piperazin-1-yl)-1,2-benzothiazole |
| Pronunciation | /θriː waɪθ wʌn paɪ.pəˈreɪ.zɪn.ɪl waɪθ wʌn tuː bɛn.zɪs.oʊˈθaɪ.əˌzoʊl/ |
| Identifiers | |
| CAS Number | [36421-15-5] |
| 3D model (JSmol) | `3D structure; JSmol model string:` `CC1=CC2=C(C=C1)NS(=O)C2N3CCNCC3` |
| Beilstein Reference | Beilstein Reference 4128787 |
| ChEBI | CHEBI:76155 |
| ChEMBL | CHEMBL18136 |
| ChemSpider | 110421 |
| DrugBank | DB12935 |
| ECHA InfoCard | 14d18beb-7e82-4499-b01e-1b7699375d86 |
| EC Number | 84166-17-2 |
| Gmelin Reference | 83455 |
| KEGG | C12008 |
| MeSH | D010576 |
| PubChem CID | 72210 |
| RTECS number | KB6477000 |
| UNII | 472C2S9B6T |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | DTXSID9046617 |
| Properties | |
| Chemical formula | C11H13N3S |
| Molar mass | 252.34 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.27 g/cm³ |
| Solubility in water | insoluble |
| log P | 1.94 |
| Vapor pressure | 2.61E-7 mm Hg at 25°C |
| Acidity (pKa) | pKa = 8.3 |
| Basicity (pKb) | 7.02 |
| Magnetic susceptibility (χ) | -78.6×10^-6 cm³/mol |
| Refractive index (nD) | 1.670 |
| Dipole moment | 4.56 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 385.15 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -22.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4474.5 kJ/mol |
| Pharmacology | |
| ATC code | N05AE04 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | > 181.8°C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 640 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 1174 mg/kg |
| NIOSH | RN 3647-69-6 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.02 mg/m³ |
| Related compounds | |
| Related compounds |
Benzoisothiazole Piperazine Risperidone Ziprasidone Lurasidone |