People who have watched the evolution of specialty chemicals since the 20th century might remember the surge in interest around imidazole derivatives—driven partly by the search for more versatile surfactants, biocides, and pharmaceuticals. Chemists looked at alkyl-imidazoles as tools with levers for tweaking lipophilicity, catalytic behavior, and biological compatibility. Among these, 2-Undecyl-Imidazole didn’t exactly get top billing, overshadowed by simpler analogs. Stepwise, with each decade, as the demand for high-performing corrosion inhibitors rose—especially within oilfield and industrial water treatment—long-chain imidazoles found a place on lab benches and pilot plants. There’s a memory from the 1980s when an uptick in antimicrobial research saw clusters of researchers adding alkyl chains to imidazole rings and noting how this affected cell membrane interactions. This compound grew from that wave of inquiry.
2-Undecyl-Imidazole consists of an eleven-carbon alkyl tail anchored to a fused aromatic imidazole ring. It stands apart from basic imidazoles, bringing an amphipathic character that makes it valuable for applications needing both hydrophilic and hydrophobic faces. The specialty chemical sector treats it less as a simple lab curiosity, more as a component for targeted tasks—from corrosion control and antimicrobial coatings, to catalyst supports and organic synthesis intermediates. Speaking from experience, procurement managers rarely see it in stockrooms of big-box bulk chemical suppliers. Instead, they reach out to specialist vendors, hinting at a smaller-scale, precision-driven ecosystem behind this compound.
At room temperature, 2-Undecyl-Imidazole appears as a waxy solid or viscous oil, depending on purity, with a faint odor that reminds you this is a byproduct of organic synthesis. It melts at a modest temperature, usually around 50 to 60°C, and dissolves more readily in alcohols or chlorinated solvents than in water. That long hydrocarbon tail seems to help in oil solubility. Chemists recognize this not just through solubility tables, but also through practical lab cleaning, where acetone wipes tackle residue while plain water barely budges it. The imidazole core, rich in nitrogen, displays basicity and forms salts with acids. It’s neither the most volatile nor the most flammable chemical in the catalog, but its low volatility and mild surfactant properties make it manageable in standard laboratory settings.
On a technical bulletin, one finds purity ratings (usually above 96%), descriptions of residual solvents, melting point range, and moisture content. Suppliers label drums with UN codes, precautionary pictograms, and hazard statements stressing eye and skin irritation. Batch numbers and analytical certificates matter to buyers, especially in pharmaceutical or fine chemical synthesis, as any impurity can behave unpredictably. Lab managers stress that real-world handling means stickers on containers may fade faster than the material itself goes out of spec, especially under frequent glove changes and solvent splashes.
Chemical synthesis most often starts with the alkylation of imidazole itself, using 1-bromoundecane or 1-chloroundecane under basic conditions. Nucleophilic substitution gets the alkyl chain onto the nitrogens, but controlling the reaction to avoid over-alkylation, tars, or side-chain rearrangement takes experience. Anyone who has performed this reaction quickly learns the importance of clean glassware, dry solvents, and deliberate dropwise addition to avoid runaway exotherms or clogging. Post-reaction, vacuum filtration and repeated washes with hexane help to isolate the target compound. Purification might involve silica column chromatography, which is as much an art as a science, especially when deciding how much eluent to run.
In synthetic labs, folks see 2-Undecyl-Imidazole’s versatility as anchored in its reactive imidazole ring. N-oxidation, acylation, and further alkylation can build more elaborate structures. That long side chain creates spacing between functional groups, which can affect reactivity—not always helpfully—especially in multi-step synthesis. Attempts to introduce selective substitutions sometimes prompt unexpected rearrangements if the reaction isn’t watched closely. This creates stories—not always happy ones—of restarts and redistillations, putting extra demands on both patience and planning in organic synthesis routines.
Chemists often encounter the alternate names: 2-Undecylimidazole, 2-(Undecyl)-1H-imidazole, or N-undecylimidazole. Product catalogs sometimes abbreviate the compound as C11-Imidazole. Patents and regulatory listings may reference CAS number 43062-71-5. Uniformity in naming helps during procurement, but practical experience says double-checking the structure remains important, since small differences—like placement of that undecyl chain—can dramatically shift both properties and regulatory status.
Operators in production or R&D facilities emphasize gloves, goggles, and fume hood work as routine practice—reports indicate repeated skin exposure can lead to irritation or sensitivity. Safety data sheets reference potential toxicity if ingested or inhaled, although acute symptoms tend to occur at unlikely exposure levels under ordinary lab routines. Proper ventilation features heavily in the design of chemical storage and benchtop setups, reflecting lessons learned from spills and splashes rather than merely ticking compliance boxes. In cases where bulk handling occurs, spill trays and chemical-resistant surfaces cut cleanup times and exposures. Emergency protocols get revisited and tested not as a bureaucratic task, but because even low-risk lab chemicals can surprise complacent hands.
The core uses for 2-Undecyl-Imidazole center on corrosion inhibition, especially in oil and gas pipelines and water treatment systems. Its surfactant properties allow it to attach to metal surfaces, forming a protective film that blocks oxidation. In industrial labs, researchers also test it as an antimicrobial additive in coatings, paints, and plastics. Some catalysis studies harness the imidazole ring’s electron-sharing tendencies for transfer hydrogenation and other transformations. Biomedical research, although more cautious, looks at this compound in the context of antifungal or antibacterial agents—though toxicity hurdles persist. Downstream users sometimes add the compound to formulations where it blends with other active ingredients, providing both protection and performance. Engineers experimenting with self-healing coatings or responsive surfaces eye it for new roles, counting on its chemical versatility.
Active research targets more sustainable production routes—using cleaner, solvent-minimized processes or greener alkylating reagents, for instance. In academic collaborations, people investigate derivatives of 2-Undecyl-Imidazole for higher selectivity in catalysis or new antimicrobial functions without crossing toxicity thresholds. Computational chemists model its interactions with cell membranes or metallic surfaces, hoping to predict behavior before hitting the bench. The push for greater efficacy without compromising environmental impact drives a lot of these efforts, as teams search for comfort between regulatory approval and real-world demand.
Toxicology teams examine acute and chronic exposure in cell cultures and animal models. Results show that 2-Undecyl-Imidazole, like many imidazoles with long side chains, can disrupt membrane function at higher concentrations. This poses a challenge for any biocidal or pharmaceutical application; efficacy in killing bacteria often carries risk for off-target toxicity. Labs document eye and skin irritation in rabbits and cell viability drop at higher doses, prompting calls for more detailed metabolism and clearance studies. Practical toxicologists push for safer alternatives or derivatives and seek exposure limits that balance user safety with the needs of industry.
Chemists and product designers watch 2-Undecyl-Imidazole for its shifting role. Blending corrosion resistance, moderate antimicrobial performance, and a manageable safety profile, it occupies a unique spot in specialty chemicals portfolios. Future work looks promising in cleaner, more efficient synthesis and in modifications that improve performance or biocompatibility. Regulatory pressure and sustainability demands keep labs chasing greener routes and less toxic derivatives. Engineers and formulators will likely test new applications—responsive coatings, advanced biomedical materials, and next-gen surfactants—pointing toward a future where the compound does more with less risk. For those who rely on it in daily work, the best solutions come from persistent, incremental improvement and unwillingness to accept ‘good enough’.
Take a stroll through the dusty back corridors of industrial chemistry and you’re bound to bump into a bunch of weird-sounding names. 2-Undecyl-Imidazole rings a bell for a handful of chemists and, interestingly, a lot of folks who care about fighting rust—real, gritty, creeping rust that eats up infrastructure when no one pays enough attention.
This compound shows up a lot when folks deal with corrosion, especially in the paints and coatings that cover bridges, pipelines, ships, and big, expensive metal things that aren't meant to crumble in the rain. Now, nobody wants to wake up one morning and find a unexpected hole in a ship’s hull or see streaks of orange crawling up a brand new overpass. The repair bills could eat through community budgets, not to mention the safety risks.
Corrosion seems like such a slow problem, but anyone who’s spent a summer scraping rust off tools or cleaning up old farm equipment knows how fast it can sneak up. 2-Undecyl-Imidazole steps in as a corrosion inhibitor. The molecules bond on metal surfaces and stop water, oxygen, and salts from getting in, which keeps that orange creep from taking over. It's a bit like putting on a rain coat, but for steel instead of skin.
Engineers in oil and gas lean on these additives because offshore rigs take a daily beating from seawater. Roads and bridges see the same abuse from salted winter slush and rain. Without inhibitors, our highways and overpasses look rough after only a couple of seasons.
There’s more to this chemical than just rust prevention. Some labs use 2-Undecyl-Imidazole to help with chemical reactions where a stable imidazole base comes in handy, but it rarely grabs headlines outside the anti-corrosion business. You probably won’t find it on a grocery store shelf, either.
There’s an environmental side, too. Lots of folks worry about what happens to all those paint flakes that wash off old buildings and ships. Many inhibitors used decades ago have left a toxic legacy. Today, there’s pressure to use compounds that protect both the metal and the water supply. I’ve seen regulatory bodies keep a close watch on new research, and companies want solutions that work without causing headaches down the road. 2-Undecyl-Imidazole is often viewed as a safer bet compared to heavy metals and older coatings loaded with chromium.
Nobody pretends the rust problem is solved for good. Testing in real weather, not just labs, matters a lot. Paint that passes every trial in a climate-controlled booth can flake off in a Minnesota winter or Gulf Coast storm. This is where conversations about price come up—newer and safer chemicals often cost more, and corner-cutting can lead to big costs later.
What needs attention is public funding and long-haul thinking. I’ve watched cities patch bridges every spring, quick-fixing what proper care could prevent. Pushing for smarter coating choices at the start makes a big difference, and choosing compounds like 2-Undecyl-Imidazole—ones balancing protection and safety—keeps metal useful longer and cuts down on waste.
Folks may not see the chemistry behind a new coat of paint, but the choices simmering in those barrels are quietly shaping public safety and budgets for decades.
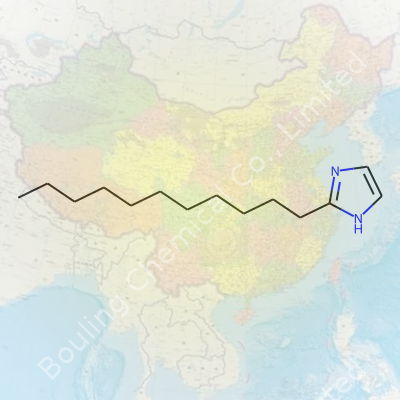
2-Undecyl-imidazole always grabs attention on a chemical board. The name hints at what makes it tick: it’s got an imidazole core, which is a five-membered aromatic ring with two nitrogens, and it’s also sporting a long undecyl chain. That undecyl part means a straight, unbranched chain with eleven carbon atoms. You're basically sticking an oily tail onto a polar, nitrogen-filled ring.
Draw that on paper, and you end up with one end that loves to mix with water (thanks to the nitrogen-rich imidazole group), and the other end that's straight up oily. The chemical formula tells its own story: C14H26N2. Ever try balancing a molecule with both grease and grit? That's what nature does with amphiphilic structures, like this one.
Compounds like 2-undecyl-imidazole make a difference far beyond the lab. That dual nature — able to mingle with both water and oil — helps when you need something to bust apart fat or form stable blends in messy, mixed environments. This opens doors in places that have to clean or coat. You’ll often find spin-offs of this structure working hard as corrosion inhibitors for metals, especially in the oil industry, where pipes and gears spend their lives battling rust and chemical attacks.
Back in graduate school, I spent long hours with structures like this, fiddling with temperature and pH. What stuck with me was how the undecyl chain always made the compounds better at protecting surfaces, almost like packing on a raincoat. If nothing stuck, the surface shrugged off water. It’s personal experience, but I remember those tests because corrosion is never a small issue for big industrial systems. Expensive pipelines fail because the chemistry isn’t right — businesses lose money, water supplies get contaminated.
Looking around the literature, there's a clear reason why formulas like C14H26N2 keep getting attention. The imidazole ring chelates metals, grabbing onto atoms and anchoring itself, while that oily tail stands guard, blocking attack by water and oxygen. This means you buy time and protection for everything underneath — in tanks, machinery, even medicine packaging.
Studies from the last decade highlight how similar molecules drop corrosion rates by huge percentages, sometimes by more than 90% in acid baths. The trick lies in fine-tuning the chain: longer chains up hydrophobicity, but make the whole thing harder to dissolve in water. In real-world engineering, balance matters: too little oiliness, the layer rinses off; too much, and the stuff clumps together, useless.
If you look past industry, features of 2-undecyl-imidazole echo through biochemistry, cleaning products, and even drug delivery. That odd ability to slip between greasy and watery worlds suggests broader uses. I’ve seen new approaches in surface coatings that borrow from this structure, building smarter, longer-lasting barriers. There’s also a push for environmental alternatives, so tweaking such molecules for better biodegradability could help clean up chemistry-heavy industries.
Getting creative with imidazole chemistry doesn’t just give us textbooks full of formulas. It lays foundations for safer machines, cleaner water, and less waste from failing infrastructure. As for the structure of 2-undecyl-imidazole, it’s all about taking the best of both worlds — stubborn metal protection and adaptability to harsh conditions. Sometimes that's what it takes to keep the real world running.
Plenty of folks scratching their heads at the name 2-Undecyl-Imidazole. The chemistry crowd calls it an “imidazole derivative,” meaning it’s a relative of molecules found in antifungals and industrial cleaners. It shows up in specialty formulations, sometimes in corrosion inhibitors, sometimes chasing mold in paints. That reach into daily products automatically raises concern: is it safe to be near this stuff?
I've spent enough time reading safety sheets in manufacturing to know the short answer: there aren’t loads of published, layperson-friendly studies on 2-Undecyl-Imidazole toxicity for humans. Regulatory lists like ECHA and EPA don’t ring alarm bells with outright bans. Still, that doesn’t clear it of harm.
Digging into imidazoles in general, repeated skin contact often leads to mild irritation. Industrial workers in chemical plants mention dryness, redness, or itching when proper gloves go unused. These symptoms usually clear up soon after washing. The lungs don’t take kindly to fine dust or aerosols of these compounds either. That dry tickle or sniffle after handling powders—experienced that plenty of times in my early lab days—often means the body’s asking for fresh air.
Chemistry likes to surprise. One imidazole can save a life as part of a medication, another stings with overexposure. The long hydrocarbon tail on 2-Undecyl-Imidazole suggests it’s designed for surfaces, not for bodies. From what tech documents share, swallow this material and you’ll get a headache and maybe worse, just like downing any industrial surfactant—nausea, burning, maybe a trip to the hospital.
The issue that gets attention in safety meetings: build-up over time. Industrial chemicals, even with “low acute toxicity,” can turn more dangerous through repeated, low-dose contact. Chronic skin exposure sometimes brings on allergies, rashes, or chemical sensitivity. Anecdotally, colleagues with long stints in production lines show these sensitivities cropping up—body remembers chemical trespass better than the memory does.
There’s the other side of the coin—what happens when this chemical enters waterways or soil. Long carbon chains don’t always break down easily. In the environmental lifecycle, what lingers in creeks has a way of winding up in animals and, eventually, people. No wide-scale studies track how 2-Undecyl-Imidazole moves through nature. But experience tells us: respect the potential for accumulation. Risk grows not with splashy accidents, but quiet routine—traces washing down drains over decades.
I keep circling back to the basics my mentors hammered home: personal protective equipment, clean-up routines, and honest labeling on containers matter more than glossy data sheets. Glove up and ventilate the space—that’s what guards hands and lungs. Emergency eyewashes belong close by.
On the corporate side, transparency matches safety. If a process uses 2-Undecyl-Imidazole or any cousin, workers deserve clear instructions about risks. Companies owe it to communities to monitor wastewater and handle disposal with extra care. Just because something lacks a big skull-and-crossbones label doesn't mean it asks for less respect.
Bottom line from real-world handling: 2-Undecyl-Imidazole doesn’t roar as the most lethal chemical in the room. That’s easy to become complacent. Yet, respect—earned from years dodging chemical mishaps—reminds us not to cut corners. Skin, lungs, and environment all benefit from that steady caution.
Every time someone cracks open a bottle of 2-Undecyl-Imidazole in a lab, the whole room trusts their smarts. Foolish mistakes with chemicals don’t just cost jobs, they cost health. This isn’t a generic talk about chemicals—2-Undecyl-Imidazole has its own quirks that put people on their toes. Years spent around labs and industry shelves taught me this: little things like a missing label or a dusty glove make all the difference.
A lot of folks ignore temperature charts until something goes wrong. This compound sticks around longest in cool rooms, right around 2 to 8°C. It hates sunlight—throw a bottle in direct light long enough, and it changes its mind about what it wants to be. I’ve seen someone once park a drum near a sunny window; the result didn’t just smell off, it made half the bench shift into clean-up mode. So, sealed containers, away from sun and away from water, really are basics, not suggestions.
Chemicals rub each other the wrong way sometimes. You don’t want acids or oxidizers anywhere close. There’s nothing dramatic about storing 2-Undecyl-Imidazole in a dedicated cabinet, preferably with some clear organization, but ignoring this saw more than a few folks running for the eyewash station over the years.
I like to be honest—nobody can “muscle through” a chemical spill without the right gear. Gloves that don’t stand up to organics, flimsy glasses instead of goggles, open-toed shoes—almost everybody’s seen one of those errors. Skin hates the stuff, eyes hate it more, and lungs might hold a grudge for days if you let vapor float around.
Fumbling reagents usually comes from rushing or working alone. Always pair up. Shortcuts like skipping fume hoods only add to the emergency stories technicians love to trade at the end of a long day. Bottles need checks for leaks or cracks—one split on the bench makes for an ugly afternoon. Good handling always means keeping paper towels, vinegar for neutralizing, and spill kits ready. All this sounds simple, but these are the first steps forgotten during busy hours.
MSDS sheets cut through the rumors. 2-Undecyl-Imidazole doesn’t set off alarms for being wildly toxic or explosive, but that’s no reason to slack off. It doesn’t belong in open containers for long; vapors stack up fast, and even a little can cause headaches or worse. Waste has to land in clearly marked chemical bins, not the trash. If disposal turns into guesswork, disasters line up right behind.
Most serious mistakes don’t come from one big error. They sneak in through everyday messes—outdated labels, incomplete safety briefings, broken vent hoods, or reversed bottles. One way forward? Daily checks. Five minutes spent walking through the storage room saves hours dealing with panic later. Supervisors and lab techs should trade notes often, not months apart.
I’ve watched how labs thrive with posters showing storage layouts, laminated safety cards by every bench, and real drills every few weeks. Complacency falls away when everyone knows what to do and where to move. Even if you think you’ve handled 2-Undecyl-Imidazole for years, that one slip when you’re under pressure can change everything, and not for the better.
Every workplace can build habits that fit their size and risks. I remember a spray bottle labeled “distilled water” that turned out to be something else entirely—it’s easy to write off these moments until small errors pile up. Stick to basics, stay alert to detail, and make backup plans part of daily life. 2-Undecyl-Imidazole sticks around labs for good reason, but so do stories of what happens when respect for rules wears thin.
Purity takes center stage the moment you set out to buy 2-Undecyl-Imidazole. In years of working in labs and dealing with chemical vendors, I’ve learned that small differences in purity can mean the world during synthesis or research. For 2-Undecyl-Imidazole, most suppliers offer a purity above 97% for advanced lab applications. Purity sometimes creeps up to 99% for pharmaceutical or biotech uses, though most industrial batches don’t make it that far.
Staring at product sheets and certificates of analysis has taught me: numbers like 97% or 99% often come with fine print. Those missing few percent usually account for water, trace solvents from manufacturing, or leftover unreacted starting materials. If you’re running a reaction with 2-Undecyl-Imidazole, these “impurities” can bite you later. High-purity grades cost more, but they often spare a lot of troubleshooting headaches.
On the market, three flavors show up most: technical, laboratory, and high-purity. I’ve ordered technical grade plenty of times for quick pilot projects or surface chemistry experiments where every last impurity doesn’t make a big difference. This grade sometimes sits around 95%, which means you’re gambling with the presence of unknowns. For sensitive research or anything headed to clinical trials, it would be reckless to settle for less than analytical or pharmaceutical grades. These higher grades might look boringly similar on paper, but the peace of mind they give can’t be overstated.
People sometimes chase the cheapest grade, hoping impurities won’t matter. Once, my team ran a screen with technical grade, and unexpected results kept popping up—traced back to a contaminant that shouldn’t have been there. We switched to a higher grade, paid more per gram, and got reproducible results. That kind of lesson sticks with you every time you look at a spec sheet.
For coatings, lubricants, or similar uses, the lower grades are hard to argue with because costs matter at scale. But the story changes fast if you’re headed into medicinal chemistry or electronics, where even the smallest impurity can throw off results, poison catalysts, or spark a recall. With 2-Undecyl-Imidazole, I’ve seen electronics companies go through rounds of in-house profiling just to make sure even a 0.5% contaminant won’t affect conductivity or stability.
Regulations also push the need for top-notch grades. The European Chemicals Agency, for example, keeps a close watch on material quality for chemicals moving into sensitive supply chains. In my own work with chemical compliance, I’ve seen companies forced to reformulate when a previously “good enough” grade no longer passes new standards.
Certainty often comes down to trusted suppliers, batch testing, and lots of transparency. In practice, I always ask for a Certificate of Analysis, and if possible, get a third-party lab to double-check things before running expensive research or production.
If budget is tight, it’s tempting to stretch for a lower grade, but any savings can disappear fast if contaminated batches halt progress. Whenever possible, source the highest grade the project can afford, and back up every shipment with independent verification.
Nobody wants to explain to their team—or their boss—why a project failed due to a chemical shortcut. Purity and grade in 2-Undecyl-Imidazole aren’t just technical specs. They steer experiments, budgets, and even careers. Trust what’s in the bottle. Your results will thank you.

| Names | |
| Preferred IUPAC name | 2-undecyl-1H-imidazole |
| Other names |
2-Undecylimidazole 2-Undecyl-1H-imidazole 2-Imidazolylundecane |
| Pronunciation | /tuː ˈʌn.dɪ.sɪl ɪˈmɪ.dəˌzɔːl/ |
| Identifiers | |
| CAS Number | 24316-93-4 |
| Beilstein Reference | 1713706 |
| ChEBI | CHEBI:87255 |
| ChEMBL | CHEMBL154760 |
| ChemSpider | 24666108 |
| DrugBank | DB08731 |
| ECHA InfoCard | 03e8ae0d-ad17-4299-bcaa-c6dab7101cc5 |
| EC Number | 223-452-7 |
| Gmelin Reference | 107757 |
| KEGG | C18596 |
| MeSH | D000081242 |
| PubChem CID | 12240311 |
| RTECS number | ZH7525000 |
| UNII | E6AL4RVF0I |
| UN number | UN3272 |
| CompTox Dashboard (EPA) | DTXSID80953572 |
| Properties | |
| Chemical formula | C14H26N2 |
| Molar mass | 292.48 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | characteristic |
| Density | 0.95 g/cm3 |
| Solubility in water | insoluble |
| log P | 3.7 |
| Vapor pressure | 0.000044 mmHg at 25°C |
| Acidity (pKa) | 6.92 |
| Basicity (pKb) | 5.42 |
| Refractive index (nD) | 1.520 |
| Viscosity | 44.1 cP at 20°C |
| Dipole moment | 3.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 537.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -83.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -6833.6 kJ/mol |
| Pharmacology | |
| ATC code | D01AC18 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| Flash point | > 116°C |
| Lethal dose or concentration | LD50 oral rat 2200 mg/kg |
| LD50 (median dose) | LD50 (median dose): 730 mg/kg |
| REL (Recommended) | 3 mg/m³ |
| Related compounds | |
| Related compounds |
1-Butyl-2-methylimidazole 1,2-Dimethylimidazole 1-Octyl-2-methylimidazole 1-Methylimidazole 2-Undecyl-4-methylimidazole |