Decades ago, interest in heterocyclic amines led chemists to focus on the thiophene ring, a five-membered structure holding sulfur in its core. Drawing inspiration from natural amino-ethyl compounds, researchers synthesized 2-thiopheneethylamine in the mid-20th century, expanding on ideas that peppered the landscape of both organic and medicinal chemistry. Much of this early work happened in academic labs eager to understand the behavior of sulfur analogs to known phenylethylamines. Gradually, the molecule earned its place in specialty chemical catalogs, used as a stepping stone for a host of molecules in both universities and pharmaceutical companies.
2-Thiopheneethylamine, sometimes paired with its abbreviation TEA, earns attention for its distinctive blend of aromatic and amine chemistry. Researchers value its structure because it offers flexibility in reactivity and avoids some pitfalls tied to similar benzene-based compounds. While not found directly in nature, the way this molecule mimics some biological structures makes it appealing for work in several areas, especially when looking to understand the effect of sulfur atoms in classic synthesis routes.
This chemical appears as a clear, oily liquid, color ranging from nearly colorless to a slight yellow tint after exposure to air. With a molecular formula of C6H9NS and a molar mass of around 127.21 g/mol, 2-thiopheneethylamine comes with a density close to 1.09 g/cm3 and a boiling point walking just above 226°C. It features a strong amine odor blended with a sulfurous hint, standing out among other common laboratory amines. Its solubility stretches into water and most polar organic solvents, helping it participate in various reaction pathways without much trouble.
Most suppliers offer this compound at purity levels of 98% or greater, often labeling ISO regulatory codes, hazard pictograms, and batch information prominently on the bottle. Users find a straightforward molecular diagram, a CAS number (1527-89-5), and shipping restrictions. Clear labeling on protective storage—dry, room temperature, shaded from light—points to its easy oxidation and sensitivity to moisture, so labs keep bottles sealed tight and transfer contents using basic PPE like gloves and goggles.
Crafting 2-thiopheneethylamine usually starts with a thiophene ring functionalized at the 2-position. Chemists often employ a two-step process: they attach an ethyl group through alkylation, then introduce the amine via amidation and subsequent reduction. Using 2-thiopheneacetonitrile as an intermediate, hydrogenation agents or lithium aluminum hydride can tackle the last step, producing the free amine. Other procedures lean on reductive amination with ethylamine and a thiophene carbaldehyde, offering high yields under mild conditions. Each method comes with a need for careful handling, especially considering the potential for exothermic reactions and by-product control.
2-Thiopheneethylamine walks a fine line between stable aromaticity and reactive nucleophilicity. Attaching groups to the amine, like acylation to make amides, provides an entry into more complex molecules. Reacting with acyl chlorides, acid anhydrides, or activated esters allows for a quick route to various thiophene derivatives. The ethyl linker handles oxidative or halogenation reactions without falling apart too easily. The sulfur in the ring gives the molecule a different set of reactivity options than typical phenylethylamines; for example, oxidation at the sulfur leads to sulfoxide or sulfone analogs, both used in specialty chemical development and as tools in metabolic study.
Labels on bottles and journal articles point to a range of synonyms: β-(2-Thienyl)ethylamine, 2-(2-Aminoethyl)thiophene, and 2-Thienylethylamine show up most commonly. Older literature sometimes bundles related compounds together, but the strict definition sticks with the amino-ethyl group sitting at the 2-position relative to the sulfur atom. CAS number 1527-89-5 tracks this substance across suppliers and regulatory records.
Amines call for respect in the lab, and 2-thiopheneethylamine is no different. It can irritate the skin, eyes, and mucosa, so gloves, safety glasses, and lab coats stay on throughout use. Good ventilation or fume hoods handle any vapor that forms, especially since the sulfur content can intensify the sharp odor and potential allergic reactions. Standard safety precautions step up if scale exceeds a few grams, adding face shields and chemical spill kits nearby. Waste gets segregated with other organic amines and bagged for licensed disposal. Shipping and storage regulations reflect its flammable and corrosive nature in concentrated form, and documentation asks for readiness during audits or inspections.
Most use of 2-thiopheneethylamine circles around drug development, specialty polymer synthesis, and agrochemical research. The pharmaceutical industry leans on its unusual structure to explore CNS-active scaffolds, since swapping sulfur for oxygen or carbon can highlight subtle bioactivity changes. Labs work on derivatives, searching for antiviral, antibacterial, or receptor-modulating properties. The molecule also makes appearances in material science, joining reactions that create conjugated polymers, giving rise to new sensors, OLED components, or environmental monitoring devices.
Developments on this amine usually involve complex synthetic planning, especially in medicinal chemistry. Research teams utilize the structure to test binding sites in enzyme inhibition or neurotransmitter analogs, often outgrowing traditional screening libraries. Chemical biologists like the way the thiophene ring interacts with protein pockets that ordinary benzene rings miss. Projects in materials science deliver new monomers, some with strong conducting properties, which advances organic electronics and light-harvesting systems. Academic groups present findings in journals that get cited for years, since the compound remains a model case for heteroaromatic amines.
Acute toxicity data on 2-thiopheneethylamine stays limited, yet patterns track alongside other simple aromatic amines. Animal studies reveal moderate systemic toxicity after ingestion or skin contact, especially at high exposures. Metabolic pathways highlight the breakdown of the ethylamine chain and oxidation at the sulfur atom, sometimes resulting in harmful metabolites if the body cannot process them quickly enough. Most protocols call for careful monitoring, especially if the compound moves into preclinical testing as part of a larger drug candidate. Regulatory authorities assign hazard codes based on both its amine and sulfur content, flagging it as potentially harmful to aquatic life as well.
Interest in 2-thiopheneethylamine looks set to grow, thanks to the upsurge in bioisosteric replacement strategies in pharmaceutical research. As companies and universities chase new scaffolds to sidestep patent constraints or avoid known side effects, sulfur heterocycles get a closer look. The compound has a shot at moving deeper into synthetic biology, especially in the areas aiming to engineer nontraditional amino acids or hybrid peptide systems. Materials science holds promise, too, with batteries and sensors demanding new conductive polymers built from aromatic amines. With green chemistry methods continuing to cut down on hazardous reagents, 2-thiopheneethylamine could earn favor as a building block in more sustainable processes, especially if suppliers scale up with lower environmental footprints and better worker protection. Leading-edge labs now explore digital tools, like AI-driven retrosynthesis, to optimize preparation and open up a fresh catalog of derivatives, potentially widening its reach well beyond today’s applications.
Some chemicals have a reputation for stirring up questions, and 2-thiopheneethylamine falls into that camp. At a quick glance, you spot the thiophene ring—something you’d expect in the wild world of organic compounds—and then you see the ethylamine tail. This setup reminds plenty of folks in the lab of compounds seen in pharmaceuticals or neurotransmitters, which immediately raises eyebrows. There’s a reason behind the curiosity. Substances like this don’t just show up in pharmacy textbooks for no reason.
Ask a chemist about 2-thiopheneethylamine’s main gig, and the answer is almost always about research. Researchers pick it for a starting block in building more complex molecules. That’s not just because it looks interesting. The structure lets scientists swap out groups or stick the molecule into bigger, ring-shaped compounds. Each tweak teaches researchers how humans or animals react to substances cousin to this one, or how the body might break them down. In the world of medicinal chemistry, these answers matter. Drug developers hunt for ways to trick stubborn diseases or tune how a drug acts in the brain, and having a Swiss-army-knife-type molecule like this is a big help.
It’s not just about building new pills, though. Walk into a materials science lab, and you'll sometimes see 2-thiopheneethylamine dropped into experiments on new polymers. The thiophene section makes these materials conduct or interact with electricity better. That finds a place in things like organic LEDs or sensors. Still, you'll see its name pop up more often in journal articles than in any household product or commercial chemical list.
Read a bit deeper into the news, and 2-thiopheneethylamine sometimes gets attention for less scientific reasons. Its shape bears a close resemblance to families of drugs folks misuse or regulate tightly. If you pay attention to the chemical’s backbone, you can spot where someone with bad intentions might tinker to skirt laws. This explains why chemical suppliers keep a close eye on anyone wanting to buy large amounts, and why regulators stay cautious.
If we're honest, most people wouldn't know this substance from table salt. But for a small group—scientists, policy folks, forensic labs—it carries a lot of weight. Key decisions sit on whether it gets new rules or if it needs tracking to stop misuse before it starts. Balancing the need for discovery against the worry about illegal drug labs gives everyone involved a lot to think about. Too tight a grip on access, and research slows down. Too loose, and law enforcement must scramble to keep up.
You can see the push and pull between curiosity and caution everywhere chemistry meets society. I’ve watched graduate students get both excited and frustrated by the paperwork around chemicals in this category. Streamlining permits for educational or research labs without opening loopholes for misuse sounds easy on paper and tough in real life. One clear solution: tighter partnerships between research institutions and regulators. Sharing why certain chemicals matter for science gives lawmakers grounds to craft smarter rules, rather than blanket bans.
Truly useful chemistry relies on transparency and respect. I’ve seen that in working labs—label everything, document every reaction, build trust brick by brick. Only by listening to both chemists and communities do we get the innovation we need, without the headaches nobody wants.
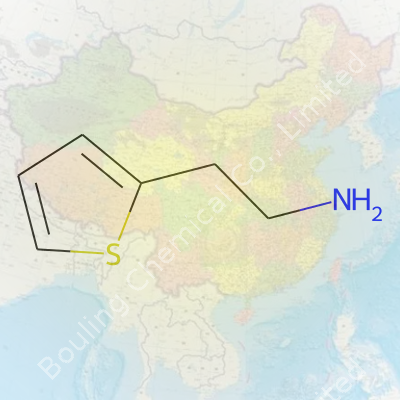
Mention 2-Thiopheneethylamine to most people and you’ll see a few blank stares. For those of us who have spent time in a lab or have an interest in chemistry, that long name gives away some clues. Here’s how it works: Start with “thiophene”—that’s a ring of four carbons and a sulfur atom, a bit like benzene’s cousin with sulfur taking the place of one carbon. Connect that ring to an ethyl chain (a string of two carbons), and tack on an amine group (-NH2) at the end. The full structure? A thiophene ring in the 2-position connected by two carbons to an amino group. Its formula is C6H9NS.
You might miss the point if you only look at this as a theoretical exercise. In my college years, our lab teacher made us build models out of plastic parts. Snapping together the thiophene ring with the ethyl group taught us what textbooks couldn’t. Chemicals like 2-Thiopheneethylamine are the raw language of many scientific conversations. They sit at the start of lists of research chemicals, and sometimes become building blocks for medicines or specialty materials.
Beyond the textbook, structure shapes everything. The sulfur atom in the ring bends the electron cloud and adds a twist you don’t see in plain hydrocarbons. That single swap changes how the molecule sticks to proteins, gets absorbed by the body, or binds to surfaces. Years later, in a pharmaceutical project, a compound carrying a similar sulfur-containing ring showed up as a lead candidate for a blood pressure drug. Chemists still spend late nights working out how those little changes can affect an entire drug pipeline.
Clever researchers put molecules like this to work in all kinds of roles. You’ll find compounds based on ethylamine groups in everything from dyes and neurotransmitter research to high-tech electronics. If you’ve ever heard of serotonin, its shape isn’t that far off from thiopheneethylamine’s core; a ring, an ethyl chain, and an amine. That enforces the thought that simple structural changes transform how a compound behaves inside a living cell or in a manufactured product.
The world of specialty chemicals depends on diversity. A tweak, like swapping a benzene for a thiophene, opens new doors. Some people see molecules as only for the realm of the specialist, but the reality, whether it’s synthesizing new drugs or building organic semiconductors, rests on exploring variations like this one.
One major concern lies in safety and availability. Not every lab can get their hands on fresh 2-Thiopheneethylamine; regulations and limited suppliers slow down research. There’s an opportunity for companies to share resources or create local sources for rare building blocks. Pollution from chemical manufacturing hits home too. Cleaning up waste streams from thiophene synthesis falls into the hands of both companies and regulators. Years of watching university and industry collaborations reach better waste management prove that pushing for greener production works, but it takes persistent cooperation.
A chemical like 2-Thiopheneethylamine asks a simple question: How do we build and use molecules responsibly? From the underlying structure to its impact on society, it becomes clear that understanding chemical basics always influences something bigger—sometimes an entire field of study, sometimes the safety of a factory worker, and sometimes the direction of tomorrow’s technology.
Research often hinges on whether a compound actually arrives in the lab—without it, protocols stall and discoveries wait. That’s true with 2-Thiopheneethylamine. This compound sits in an awkward place. It’s not listed as a household chemical, but it keeps popping up on the radars of experimental chemists and pharmaceutical developers. Someone with a clear research plan, and the right paperwork, might search for it in catalogs from Sigma-Aldrich, Alfa Aesar, or any of the other global suppliers. Sometimes, 2-Thiopheneethylamine shows up with a neat product code, price tag, and purity figure. At other times, there’s just a “not available” sign or a prompt to send an inquiry.
Researchers trying to set up a project using this compound might hit a wall, especially if the substance’s status shifts. Supplies of specialty chemicals don’t just depend on science—they are tangled up with regulations, shipping rules, and raw material shortages. A supplier’s website may offer a purity specification, but that offer can vanish without warning if regulators clamp down, or if borders tighten. I’ve seen labs shift entire projects over a chemical suddenly stuck in customs or taken off the shelf because of new rules.
This molecule interests scientists for its role in synthesizing more complex compounds. It acts as a building block for research into materials science, pharmaceuticals, and even sensor technology. Its structure, with both an amine group and a thiophene ring, lets chemists push into different directions. There’s talk of new drug scaffolds and optical sensors starting from just this backbone.
What complicates the story comes from a different world—a blend of regulatory oversight and public safety. Compounds with structures similar to 2-Thiopheneethylamine sometimes show up on controlled substance lists, thanks to their relationship with psychoactive derivatives. A molecule can shift from “promising” to “problematic” overnight, based on changes in drug laws or new designer substances appearing on the street. For researchers, constant document checks and compliance headaches can slow everything down.
My own experience with obscure chemicals taught me that persistence isn’t always enough; connections and paperwork matter just as much. If a research team needs 2-Thiopheneethylamine, reaching out directly to multiple suppliers often shakes something loose. Some companies only sell to registered institutions, and proof of research intent lands on their desks before they ship anything. Labs should get used to completing end-user declarations and reading shipping terms line by line.
For those who truly need this compound, building a collaborative network helps. Contacting colleagues overseas, partnering with universities, or even working with compound libraries keeps the search moving. At one point, I teamed up with a research group abroad to pool chemical resources. We traded paperwork, emailed customs, and cracked the logistics together.
Researchers might also turn to custom synthesis firms, though that route gets expensive. Some firms, often based in India or China, quote prices per gram and produce small batches. There are risks—slow delivery, quality issues, and the nagging worry about legal compliance. Anyone who chases a rare compound learns fast that one lost shipment or a single customs holdup can wipe out months of planning.
Lab work often means navigating complexities outside the chemistry itself. Advocating for better international cooperation around research chemicals stands to help both innovation and safety. Suppliers who bridge the regulation gap, and researchers who band together, can ease the process. The lesson—dig deep, stay connected, and always, always read the fine print.
Looking at the label on a bottle of 2-Thiopheneethylamine tells you only part of the story. Lots of labs stack hundreds of chemicals, but not all of them get the attention they deserve. Small molecules like 2-Thiopheneethylamine might seem harmless at a glance, but anyone who’s managed a cluttered chemical shelf knows otherwise. This compound, with its strong amine smell, tends to get tucked behind bottles until someone realizes it’s degraded or leaking fumes.
Walking into a storeroom that’s been around for a while, I’ve seen clear signs of trouble. Caps get loose, labels fade, humidity sneaks in. Just last winter, a bottle frozen to the back of a cold room had cracked—luckily, someone had double-bagged it. That’s experience talking. Chemicals don’t stay stable by magic. For 2-Thiopheneethylamine, the smartest approach is to keep it in a cool, well-ventilated space, away from light and heat. Most folks forget that even low light in a storeroom can trigger photo-oxidation over time. It’s easy to overlook, but years of mistakes drive home the point.
Oxygen and moisture from the air creep in each time you open a bottle. A tight seal helps but doesn’t completely solve the problem. I’ve learned it pays off to decant small amounts into smaller vials for daily use. That routine cuts down exposure and protects the main supply. In busier setups, keeping the bulk container under nitrogen isn’t extreme at all—it’s practical. Moist air leads to polymerization and contamination, and then that batch won’t give reliable results in syntheses or bioassays.
Heat causes more trouble than anyone wants to believe. The temptation to leave a bottle on a bench overnight makes sense in a rush, but direct sunlight or warm conditions quickly speed up undesirable reactions. Refrigeration slows things down, giving you a longer shelf life and reducing pressure buildup in the bottle. At room temperature, you’ll notice color changes and cloudiness a lot sooner. That isn’t just ugly—those changes reflect real chemical shifts.
Some folks treat storage as an afterthought, but in practice, safety grows out of smart routines. Label everything with the date received and opened, so you don’t forget what’s fresh. Segregate amines like 2-Thiopheneethylamine from strong acids, oxidizers, or peroxides. Accidental mixing has set off more than one fire or toxic plume. Using secure containers cuts waste and protects colleagues. Once, someone stored an amine in an acid cabinet; the fumes damaged expensive equipment and meant an afternoon of cleanup nobody enjoyed. You’ll only make that mistake once.
Routine checks help a lot. Twice a year, I pull everything out and inspect the inventory. Anything questionable gets disposed of following current environmental rules. Keeping an updated chemical list forces accountability. Digital logs have made it easier, but you still need a sharp eye for details. Labs with strong storage habits rarely have major incidents—and when something does go wrong, the scale stays small. If anything, better habits start with small steps: sealed bottles, cooler temperatures, and plenty of labels. Then, when you reach for that bottle months later, you’re not holding your breath in suspense.
I once worked in a small research lab where every day felt like a juggling act with different chemical substances. Among those, aromatic amines always deserved extra respect, and 2-Thiopheneethylamine fell into that basket. The bottle even looked intimidating, tucked away behind double-locked glass. After a close call with a much milder amine, I learned quickly: a few basic precautions can head off trouble.
This compound shows up in organic synthesis and pharmaceutical research, often as a building block. Its structure packs in two big concerns: amine groups tend to be reactive, and the thiophene ring hints at volatility and possible toxicity. The sniff test is a terrible idea. Exposure can irritate the respiratory system and skin, and accidental splashes have caused more than one lab to stop for an emergency eyewash.
Let’s face it, chemical-resistant gloves pay for themselves every time. I prefer nitrile over latex. Throwing on a lab coat and a reliable set of goggles makes good sense—no exceptions, not even for a quick pipette. Any direct contact might cause burns or allergic reactions. For a compound that can mess with your airways, a fume hood is the only place I would ever open a bottle or transfer a solution. Proper ventilation doesn’t just dilute fumes; it keeps everyone safe, especially if you’re working with several volatile chemicals in the same room.
More than once, I watched colleagues rummage through poorly labeled shelves. In one case, a mix-up led to a dangerous spill. Marking containers with the chemical’s full name and hazard information seems obvious, but it saves headaches. I store this compound out of direct light inside a tightly sealed bottle, preferably behind a secondary container to contain leaks. It also helps to have absorbent materials nearby for emergencies.
Someone once dropped a sample right outside the fume hood. I’ve learned to keep a spill kit stocked and close. Sand or vermiculite works for absorbing liquids—never use paper towels, which can spark more trouble. Wearing gloves, I gently scooped the mess and dumped it in a sealable hazardous waste container. Clean-up almost always takes longer than you think, and rushing can make things worse.
Disposing of 2-Thiopheneethylamine wastes through regular trash bins shouldn’t cross anyone’s mind. Local hazardous waste streams might sound complicated, but they keep toxins from returning through water or soil. I always log what I throw away and discuss with the safety coordinator before booking a pickup. Sometimes it requires dilution or neutralization steps, depending on local guidelines.
I’ve seen people brush off the dangers that come with research work, but ignoring protocols often lands folks in uncomfortable situations. Sharing knowledge about these risks helps newer colleagues develop a respectful routine. Simple steps—wearing gloves, ventilating your work area, proper labeling, careful storage, and knowing emergency procedures—help keep everyone out of harm’s way.
| Names | |
| Preferred IUPAC name | 2-(Thiophen-2-yl)ethan-1-amine |
| Other names |
2-(2-Thienyl)ethylamine 2-Thiophen-2-ylethanamine |
| Pronunciation | /tuː θaɪ.oʊˌfiːnˌiːθɪləˈmiːn/ |
| Identifiers | |
| CAS Number | [4565-36-2] |
| Beilstein Reference | 1209243 |
| ChEBI | CHEBI:34683 |
| ChEMBL | CHEMBL131700 |
| ChemSpider | 126861 |
| DrugBank | DB01985 |
| ECHA InfoCard | 100.119.520 |
| EC Number | EC 621-554-6 |
| Gmelin Reference | 78037 |
| KEGG | C06181 |
| MeSH | D017901 |
| PubChem CID | 162151 |
| RTECS number | KL2975000 |
| UNII | M75U741QT4 |
| UN number | NA1993 |
| CompTox Dashboard (EPA) | DJ4AX94JRK |
| Properties | |
| Chemical formula | C6H9NS |
| Molar mass | 143.23 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | amine-like |
| Density | 1.14 g/mL at 25 °C(lit.) |
| Solubility in water | Soluble |
| log P | 1.47 |
| Vapor pressure | 0.227 mmHg (25 °C) |
| Acidity (pKa) | 9.78 |
| Basicity (pKb) | 8.79 |
| Magnetic susceptibility (χ) | -63.3·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.605 |
| Viscosity | Viscous liquid |
| Dipole moment | 1.59 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | S⦵298 = 312.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -48.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4806.6 kJ/mol |
| Pharmacology | |
| ATC code | N06BX10 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 64°C |
| Autoignition temperature | 405 °C |
| Lethal dose or concentration | LD50 (rat, oral): 200 mg/kg |
| LD50 (median dose) | LD50 (median dose): 200 mg/kg (rat, oral) |
| PEL (Permissible) | No PEL established |
| REL (Recommended) | 50-200 mg |
| Related compounds | |
| Related compounds |
2-Thiopheneacetic acid 2-Thiopheneethanol 3-Thiopheneethylamine Thiophene Tryptamine |