Curiosity about sulfur-containing heterocycles started fueling modern chemistry more than a century ago, yet focused work on 2-thiopheneethanol really ramped up during the growth of the chemical industry after World War II. Researchers looking for new scents, flavors, and pharmaceuticals experimented with thiophene derivatives, following up on curiosity sparked by the natural occurrence of thiophenes in coal tar. Over decades, as organic chemistry labs kept growing smarter, 2-thiopheneethanol gained a reputation as a useful intermediate. It isn’t a superstar in textbooks, but many chemists have handled it during synthesis projects, flavor formulation, or even when troubleshooting a persistent odor in the lab.
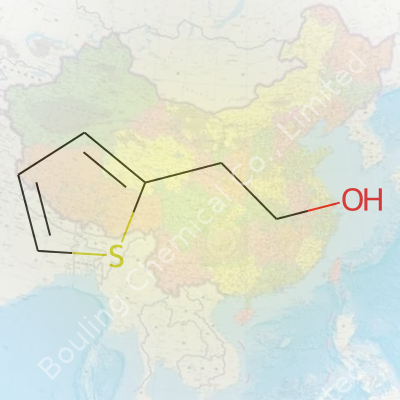
2-Thiopheneethanol—sometimes labeled as 2-(2-hydroxyethyl)thiophene—brings together an aromatic sulfur ring with a primary alcohol arm. Suppliers target this compound to both research and industry buyers, knowing how it bridges basic research and practical formulations. One bottle might go to a perfumery for a new fragrance, another to a pharma lab hunting for specialized intermediates. The structures of thiophenes unlock both scent and reactivity, and having a good handle on this ethanol derivative opens plenty of doors for applied chemistry.
Here’s what stands out after working with 2-thiopheneethanol: it pours as a colorless-to-pale yellow liquid and carries a distinct grassy or slightly toasted aroma, thanks to that thiophene ring. The compound’s melting point sits below room temperature, while the boiling point hovers around 233°C. Not everybody enjoys the scent, but once you smell it, identification gets easier. Water solubility falls on the modest end; ethanol and organic solvents do the trick far better. In the flask, the molecule stays fairly stable, but given enough time and oxygen, oxidation creeps in and the color may darken. On the chemical side, the alcohol group enables a variety of transformations, while the thiophene ring resists harsh conditions better than its oxygen cousin, furan.
Every decent supplier includes purity—usually above 98%—plus batch-specific data like GC trace, water content, and storage guidelines. Proper labeling isn’t window dressing: clear hazard symbols, recommended handling procedures, and UN numbers show thought for daily users. CAS Number 5402-55-1 sticks to this compound, though not everyone on the lab bench reads that off the bottle. Real-life handling means knowing that even small impurities or oxidation can affect downstream reactions, especially for fragrance and pharma. Suppliers with credibility win repeat customers by keeping these labels accurate, up-to-date, and honest.
Labs usually prepare 2-thiopheneethanol by reducing 2-thiopheneacetaldehyde, often relying on sodium borohydride for a straightforward transformation. Industry might run a Grignard route or reduce the corresponding carboxylic acid, choosing methods proven to scale without fouling equipment. At home in an academic lab, most students find the reduction method quick and reliable, making purification a manageable task. The simple structure means side products rarely confuse analysts, especially once the characteristic smell confirms success. On the industrial scale, keeping yields high and controlling waste bring their own challenges, and that’s where process engineering earns its keep.
That alcohol handle makes 2-thiopheneethanol appealing for etherification, esterification, and conversion into other functional groups—such as halides, tosylates, or amines. Old-school organic labs often demonstrate SN2 reactivity with it. The thiophene ring itself tolerates mild halogenation or cross-coupling, especially Suzuki or Heck reactions, giving med chemists a reliable scaffold. Adding protecting groups, making new linkers, or just using it as a building block come with relatively straightforward protocols. I’ve seen more than one graduate student breathe a sigh of relief after a textbook reaction using this compound came through the TLC analysis with a clean spot.
Label confusion often crops up, since 2-thiopheneethanol wears a few hats: it shows up as beta-thienylethanol, 2-thienylethanol, or even as 2-(2-hydroxyethyl)thiophene depending on the catalog or country. CAS registry number 5402-55-1 delivers more certainty than a product name, especially when shipping cross-borders. Chemical safety documentation might add a few more descriptors, but clear labeling helps avoid mistakes in stockrooms or on websites.
Safety data sheets recommend gloves, goggles, and handling under a fume hood due to its potential for mild skin and respiratory irritation. Compared with phenol derivatives or more volatile solvents, 2-thiopheneethanol ranks as less aggressive, but nobody enjoys direct contact. I once caught a whiff during a filtration step and can attest that even modest exposure can linger in the nose or on the skin. Fire risk remains moderate, and spills generally clean up with absorbents and ventilation. Shipping and storage must follow regulations for both flammable and toxic materials, even if the acute hazards aren’t dramatic.
Industry uses 2-thiopheneethanol as a building block in pharmaceuticals, flavors, fragrances, and agrochemicals, thanks to both its reactivity and aroma. Fragrance formulators blend it for earthy or green notes in perfumes and aromas for tobacco or food. Medicinal chemists explore the molecule as both a synthetic intermediate and a backbone for antifungal, antibacterial, or CNS-active compounds. Material science doesn’t ignore it either; some conductive polymers spring from thiophene chemistry. My time in research labs taught me that even if a compound doesn’t star in literature, its flexibility in routine synthesis wins plenty of sales for chemical suppliers.
Chemists interested in green synthesis keep testing new catalytic methods to make and modify 2-thiopheneethanol more efficiently, aiming to cut down on toxic reagents and waste. Pharmaceutical research wants broader, high-yield modifications, turning the molecule into active drugs with less fuss. Fragrance houses seek purer forms, knowing that subtle impurities can twist a formulation’s final scent. Analytical chemists build sharper detection tools, seeking better ways to quantify trace levels in complex matrices. Everything circles back to scale, purity, and cost, since budget limits often squeeze the most interesting experiments.
Published animal studies offer basic reassurance: acute toxicity lands low to moderate, but chronic effects have seen limited review. Without robust clinical data, regulatory agencies stick with caution, and I always follow best practices for containment, even if personal experience suggests low risk. Inhalation and skin exposure often result in irritation, so spills and splashes still prompt a quick trip to the sink. Waste handling follows standard procedures for organic solvents, protecting both lab staff and the downstream environment. For now, toxicity research stays ongoing, and any claim of safety gets a careful footnote in serious reporting.
The ongoing drive for green chemistry will push chemists to improve both raw material sourcing and the process efficiency of 2-thiopheneethanol synthesis. Expanding demand in pharmaceuticals, fine chemicals, and fragrance suggests that clever catalyst design and process intensification will gain ground. Improved toxicological knowledge could help certain applications move out of the research zone into wider commercial use. Meanwhile, as the flavor and fragrance industry stays hungry for new sensory notes, the scent of this sulfur ring catches more attention, keeping researchers curious about new blends and how subtle modifications might unlock fresh commercial potential.
People see complicated chemical names and check out mentally. 2-Thiopheneethanol doesn't exactly roll off the tongue, and to most, it looks like pure science jargon. I get it — it used to intimidate me, too. But looking past that, these molecules shape industries, change how perfumes smell, and even play a role in new medicines. The formula is C6H8OS, and its backbone comes with a structure that's easy to picture once you know the tricks.
Let's pick it apart. Think of a thiophene ring — a five-membered ring where four are carbons and one is a sulfur atom. That sulfur swaps in where oxygen usually goes in related rings like furan. Now, tie an ethanol group (-CH2CH2OH) to the ring at the second position. Scientists call this the "2" spot, right next to the sulfur. That’s the full story of its chemical structure:
The actual arrangement matters. Those positions decide how chemicals interact, what smells they carry, and even if they can help build new medical treatments. Years ago, I worked on an undergraduate project comparing sulfur-containing organics. What stood out wasn’t just their peculiar smells, but how that sulfur unlocked possibilities standard benzene couldn’t reach.
I once spilled a drop of 2-thiopheneethanol in a lab. Instantly, a woody, earthy aroma hung in the air. Some people compare it to roasted coffee or the subtle edge of fine whisky. That unique smell packs a punch in flavorings and fragrances. The chemical structure, with its ethanol "tail," lets it dissolve in both water and oil — a rare mix. That gets perfumers and food technologists excited.
Chemical properties grow out of structure. The sulfur’s position lets it play the middleman in reactions. For medicine, that means synthetic chemists can tweak the molecule at lots of spots, building custom drugs from the core. Pharmaceuticals always want chemicals easy to modify. 2-Thiopheneethanol gives that flexibility without being as volatile as some sulfur relatives.
No molecule comes without baggage. With 2-thiopheneethanol, overexposure can irritate mucous membranes or skin. In my early lab days, gloves were non-negotiable. The bigger picture circles back to how these aromatic organics are produced. Most still rely on petrochemical feedstocks. The shift toward greener synthesis matters; bio-based routes aren’t just a trend, they’re a necessity. Chemists look at microbial factories and plant-derived alternatives — all starting with a deep understanding of the molecule’s structure.
For progress, education wins. People working with chemicals need clear labeling, and small labs benefit from real-world safety examples. On the industrial level, supporting research into renewable production and recycling of thiophene derivatives could make a real dent in waste and pollution. Every little tweak in the chemical structure has an impact. The trick isn’t just knowing the formula, but understanding what that means in everyday use, ethics, and the way science moves forward.
Walk through a fragrance lab or step inside a flavor company, and you’ll likely bump into 2-Thiopheneethanol. This sulfur-containing compound pops up most in the creation of perfumes and food flavors. The reason? It brings a unique, earthy yet mildly sweet scent that reminds some people of roasted nuts or popcorn. Take a look at chocolate and coffee formulations—this molecule helps push those deep, toasted notes that make the aroma linger in the air after brewing a fresh cup of coffee.
As someone who once worked in a flavor house blending compounds all day, I can tell you: a teeny splash of this stuff does a lot. Natural flavors like cocoa come alive, and imitation rum or whiskey lean on it for that edge. Perfume chemists use it to anchor fruity or floral blends, letting other notes stand out without turning flat or simple. The same sulfur vibe that works in wine (think earthy whites) does wonders in both gourmet confections and colognes.
This compound doesn’t get the spotlight in new drug launches, but it does help make certain active pharmaceutical ingredients. Chemists use 2-Thiopheneethanol as a handy building block when piecing together complex molecular structures. The alcohol group and the thiophene ring offer spots for further chemical reactions—so this relatively simple molecule helps assemble antibiotics, antivirals, and even agricultural chemicals.
Sometimes, researchers discover that 2-Thiopheneethanol derivatives show mild anti-fungal or anti-bacterial effects on their own. While these uses don’t headline medical conferences, they hint at untapped potential. If funding and curiosity line up, it could spark new synthetic pathways in drug discovery, shrinking development costs.
A lesser-known application comes from the world of advanced materials. Scientists mix 2-Thiopheneethanol into polymers and electronic materials, chasing better conductivity or stability. The sulfur atom does more than add aroma—it tunes the electronic structure of new plastics, sometimes leading to better sensors or more flexible screens. Electronics rarely come up in everyday conversation, but someone crafting OLED displays or organic circuit boards may owe a small thanks to this compound.
Green chemistry circles eye this molecule because of its availability from bio-based sources. Pulling it from renewable feedstocks rather than petrochemicals could cut manufacturing’s carbon footprint, especially if demand for biodegradable plastics or low-impact electronics continues to rise.
On the job, handling 2-Thiopheneethanol isn’t as loose as it used to be. Regulatory agencies set limits for use in both food and fragrances, aiming to avoid excessive exposures. The sulfur atom can trigger reactivity, and large quantities might cause respiratory or skin irritation. My own experience in a lab taught me to respect even small volumes—safety glasses, gloves, and good ventilation matter. Ingredient tracking and clean records round out safe practices.
Replacing some uses with other sulfur compounds might dodge stricter rules, but that could mean losing out on flavor or scent quality. Ongoing studies push for clearer answers: how much exposure is too much, and what green alternatives stand up in real-world products?
In the end, 2-Thiopheneethanol keeps showing up in places where chemistry and creativity meet—each field adapting its quirks for new uses as science and industry evolve.
2-Thiopheneethanol plays its part in many labs and manufacturing settings, often popping up in the making of pharmaceuticals and dyes. The smell cuts through the air, hinting at its strong nature. If you’ve spent time around chemical stockrooms, you’ll know how even a minor breach with an organic solvent can fill the room with odors or, worse, eat through gloves in an hour. While plenty of folks get used to the glass bottles and chemical names, that whiff should always serve as a reminder to pay sharp attention. Skipping precautions once can cost you more than a ruined batch — think skin irritation, or something much more serious like a trip to the ER after an unexpected spill.
Every time I open a chemical cabinet, I check labels and lids. It’s easy to get sloppy, especially during a busy day, but keeping 2-Thiopheneethanol in containers with tight seals makes all the difference. Plastic stoppers? Forget those. Go for glass or high-quality Teflon. In my experience, low shelves beat high shelves — less risk of bottles taking a nosedive. Temperature changes can be a silent enemy; this compound stays most stable in a cool, dry spot. I've seen colleagues store similar chemicals on high-up shelves near sunlight "for convenience." That shortcut brings nothing but unnecessary risk.
This is not the stuff you stash in a closet next to office supplies. Set up in a well-ventilated chemical storeroom. I remember walking into an office where a leaking sample bottle sat on a regular desk overnight; by morning, everyone had a headache, and housekeeping ended up calling the safety officer. Flammable vapors linger in still air. Good ventilation, along with fire extinguishers and no open flames, becomes a basic expectation, not an extra.
Gloves, goggles, and lab coats — these things save skin and eyes. Nitrile gloves usually work well, but check the safety sheet and see which glove material matches best; I’ve met more than one old-timer who swore by a certain brand until the day a chemical split right through it. Contaminated clothes don’t stay in the building. Wash up after each experiment. Splashback happens: use fume hoods, not open benches, for transfers. In smaller labs, I’ve seen people pour compounds without eye protection — don’t join that club.
Many incidents start with a mystery bottle or faded label. Regular checks stop this before it happens. Sorting bottles by compatibility avoids weird reactions. I once spotted a bottle of peroxide next to organic solvents; a labmate fixed it before trouble started. Spills aren’t the end of the world with the right spill kit nearby. Baking soda, absorbent pads, and a plan for hazardous waste disposal keep things safe and tidy. Don't mix chemical waste with regular trash — one slip makes for a disaster story people tell for years.
Be mindful about disposal. Solvents like 2-Thiopheneethanol rarely go down any sink. I’ve learned to check labels and local rules every time. Separate containers for solvents and regular trash make all the difference during a busy afternoon. Bottles labeled “waste” and sealed right get picked up by hazardous waste teams, not janitors guessing what's inside. Half the time, safety comes down to respecting these routines—every day, every bottle, every label.
Buying chemicals for the lab doesn’t have to feel like hunting for hidden treasure, but with compounds like 2-Thiopheneethanol, you’ve really got to read the fine print. Purity makes or breaks any experiment, especially in organic synthesis or when making anything for pharmaceutical research. Over the years, I’ve seen chemists jump between suppliers only to find the grade isn’t as advertised or the paperwork doesn't quite match the actual sample. The trouble is, minor contamination in a so-called “high purity” bottle can throw off months of work. In the world of chemicals, no one wants surprises.
Most suppliers push 2-Thiopheneethanol in purity grades labeled as 97%, 98%, or 99%. Sigma-Aldrich, Alfa Aesar, and TCI are a few brands you’ll see popping up in catalogs. These specs sound reassuring, but it boils down to what sits in that last pesky percent. Most manufacturers support their claims with GC assay data, showing the substance usually lands in the 98% to 99% purity range. The difference between these numbers can stack up in sensitive syntheses, especially when that fraction might include water, traces of starting material, or other sulfur-containing organics that could act up in a reaction.
I remember distinctly one time in grad school: a colleague grabbed an off-brand bottle boasting “>98% purity.” Everything started off fine, no strange odors, no cloudiness, but his NMR came out with a nasty set of unidentified peaks. Turns out, there was residual tetrahydrofuran from a lazy distillation. That single incident stressed how crucial real and reliable purity data is, along with handling protocols to avoid new contaminants (especially if the bottle has been opened a few too many times in humid weather).
A chemist designs every synthetic route with the target molecule and side reactions in mind, not expecting extra surprises from the starting materials. For something like 2-Thiopheneethanol, commonly used to build pharmaceuticals, agrochemicals, and optoelectronic materials, dirty input gets in the way. Watching people spend more energy purifying their “reagent” than making the target compound leaves a sour taste.
According to catalog entries, most commercial lots top out well above 97%. TCI lists it at 98.0% minimum, Sigma-Aldrich offers 98% and 99% variants. You might pay extra for 99+%, but avoiding trace impurities gets more important as the product’s application veers into bioactive or optical domains. For basic organic synthesis, 97–98% works fine as long as you’re mindful of potential noise in the final product. It’s a case of matching quality with need, plus checking the certificate of analysis.
Double-checking the latest lot analysis before purchase saves headaches down the road. I always ask for recent analytical results. Buying 2-Thiopheneethanol from a reputable source cost a few more dollars, but I dodge the hassle of re-distillation, additional drying, and re-purification that can cost so much more in project delays. In smaller labs without a full analytical suite, relying on trusted suppliers makes all the difference.
Labs with sensitive applications sometimes distill it again or use chromatography in-house to remove traces of water or other organosulfur compounds. A little vigilance here goes a long way. For anyone ordering a new bottle, read that certificate at least as closely as the reaction protocol—because purity isn’t just a number; it’s the neglected step that either sets up success or doubles your work.
Most people haven’t heard of 2-Thiopheneethanol. Yet, it pops up now and then in places like flavor chemistry labs, fragrance production, and the pharmaceutical world. The first time I walked by a classroom where someone opened a sample bottle, the strong, slightly earthy scent stuck with me. After that, I started reading up on what that chemical does—and more importantly, if it means trouble for people around it.
Handling chemicals always brings a level of risk, and this one is no different. If you’ve ever gotten 2-Thiopheneethanol on your skin, you know it can cause some irritation. Repeated contact feels worse. The liquid seeps into cuts or cracks on your hands and, after a while, that irritation begins to sting. The safety data sheet, something all scientists keep nearby, lists eye irritation too. Once, a lab partner got a tiny splash near their eye, and the discomfort forced them to the nearest eyewash station.
Breathing in vapors can bother your nose and lungs. I only needed to work near an open flask once without a mask to realize the warning signs are there for a reason. Sore throat, watery eyes, and that raw feeling after even a minute or two in the air without good ventilation. As someone who’s accidentally taken a whiff, the body tells you fast that something’s up.
In addition to its irritating qualities, this chemical also brings a flammability risk. It catches fire at relatively low temperatures, meaning a simple spark or forgotten hotplate can create a serious problem. I watched a fire drill go sideways as students scrambled to cap containers and move them from stovetop hot plates. Since then, it’s been a lesson that the storage area should always stay cool and away from spark sources, just like the manuals say.
Chemicals don’t move from lab to factory or to a processing plant without a network of regulations. 2-Thiopheneethanol is no different, even if it doesn’t grab headlines in the way bigger, more notorious toxins do. In the United States, the Environmental Protection Agency (EPA) keeps lists of chemicals flagged for toxicity or environmental risk, and this one lands among “hazardous substances.” Transport rules, labeling, and reporting obligations apply under OSHA and EPA guidelines—if a spill happens over certain amounts, the company must notify the authorities.
Globally, the European Chemicals Agency treats it seriously too; it appears on their registries with hazard statements about skin and eye irritation, flammability, and environmental risks. Every bottle needs a clear label and Signal Word, usually “Warning,” demanding that handlers don gloves, goggles, and lab coats.
Most issues come down to preparation and respect. Students and lab techs can’t treat this compound the same as kitchen vinegar. Spills don’t always look dramatic, but cleanup must follow strict steps: use absorbent material, seal the waste, ventilate the space, and wash exposed skin immediately. Wearing gloves and goggles never feels optional. Good ventilation systems—like working in a chemical fume hood—turn hazardous tasks into safer ones.
It’s easy to blame regulators for endless paperwork, but the rules make sense after seeing even a small accident. Regular safety drills and proper equipment help, but so does a workplace culture that treats these risks seriously. Training newcomers, reviewing protocols, and sharing close calls—these steps often keep trouble from turning into something far worse.
| Names | |
| Preferred IUPAC name | 2-(Thiophen-2-yl)ethan-1-ol |
| Other names |
2-Thienylethanol 2-(2-Thienyl)ethanol beta-Thienylethanol Thiophene-2-ethanol |
| Pronunciation | /tuː θaɪ.oʊˈfiːn.iːˌθæn.ɒl/ |
| Identifiers | |
| CAS Number | 5402-33-7 |
| Beilstein Reference | 1202201 |
| ChEBI | CHEBI:132588 |
| ChEMBL | CHEMBL13361 |
| ChemSpider | 12718 |
| DrugBank | DB14096 |
| ECHA InfoCard | 03c7be0d-6b06-426a-972a-ab533f93d811 |
| EC Number | 2.3.1.28 |
| Gmelin Reference | 9444 |
| KEGG | C06223 |
| MeSH | D016207 |
| PubChem CID | 11749 |
| RTECS number | KL5950000 |
| UNII | 622ZI5D6WY |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | DTXSID1022231 |
| Properties | |
| Chemical formula | C6H8OS |
| Molar mass | 114.18 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | aromatic |
| Density | 1.129 g/mL at 25 °C |
| Solubility in water | soluble |
| log P | 0.88 |
| Vapor pressure | 0.0078 mmHg (25 °C) |
| Acidity (pKa) | 14.3 |
| Basicity (pKb) | 15.09 |
| Magnetic susceptibility (χ) | -61.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.569 |
| Viscosity | 2.229 cP (20°C) |
| Dipole moment | 2.07 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 293.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -26.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3731.3 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P305+P351+P338, P370+P378 |
| NFPA 704 (fire diamond) | 1-2-0-W |
| Flash point | 100°C |
| Autoignition temperature | 270 °C |
| Explosive limits | 1-8.6% |
| Lethal dose or concentration | LD50 (oral, rat): 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 1740 mg/kg |
| NIOSH | PB6480000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0-2°C |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Thiophene 2-Thiophenecarboxaldehyde 2-Thiopheneacetonitrile 2-Thiophenemethanol 3-Thiopheneethanol |