The journey of 2-thiophen-2-ylpropanoic acid traces back to the deep roots of organic synthesis in the early twentieth century, when scientists grew increasingly interested in sulfur-containing heterocyclic compounds. Chemists looked at five-membered rings like thiophene, driven by curiosity about their aromaticity and potential in fields ranging from dyes to drug discovery. As the synthetic tools evolved through decades, the development of propanoic acid derivatives added a new chapter. Laboratories equipped with improved analytical techniques—NMR, chromatography, mass spectrometry—helped researchers confirm molecular structures with greater certainty than their predecessors working mostly with melting points and boiling points. With industry’s growth, both pharmaceutical and materials sectors saw expanded interest in these compounds, solidifying 2-thiophen-2-ylpropanoic acid’s position as a notable player among thiophene derivatives.
2-Thiophen-2-ylpropanoic acid combines the thiophene ring—a sulfur-containing aromatic system—with a propanoic acid side chain. This creates a molecule valued in fine chemicals research and as a scaffold for drug synthesis. In the chemical market, it typically appears as a solid or crystalline powder, appreciated both for its reactivity and as a flexible intermediate. Its relatively simple structure allows it to serve as a building block for more complex molecules, offering synthetic chemists a starting point for diverse transformations.
Chemically, the compound presents a molecular formula of C7H8O2S, packing a distinct profile. The presence of the thiophene ring brings aromatic stability and a modest electron-withdrawing character due to sulfur. The propanoic acid group not only grants solubility in polar solvents but also introduces moderate acidity (pKa typically around 4–5 for carboxylic acids). At room temperature, this compound usually appears as a colorless to light yellow solid with a melting point near the mid-hundreds Celsius range. Odor-wise, it might carry a mild, sulfuric tinge, something that isn’t unusual for thiophene derivatives. The compound dissolves efficiently in common solvents like ethanol, methanol, and dimethyl sulfoxide (DMSO), which plays a role in both its lab handling and subsequent chemical transformations.
In laboratories and industrial settings, specifications for this product often cover purity grades (usually upwards of 98%), moisture content, melting point confirmation, and residual solvents. Labels indicate hazard statements dictated by global harmonized standards, such as “Irritant” or “Harmful if swallowed,” and they display chemical identifiers like CAS number, batch number, and expiration. The chemical’s shelf-life hinges on packaging—airtight containers barred from strong sunlight or excessive moisture keep it stable for months, sometimes years. Documentation packet includes certificates of analysis, safety data sheets, and, depending on country regulations, transport hazard statements to ensure handlers know exactly what they’re working with.
Preparation often begins with the Friedel-Crafts alkylation of thiophene, followed by carboxylation steps. One classic strategy involves starting from 2-alkyl thiophenes undergoing oxidation and subsequent conversion to the acid via hydrolysis. Chemical suppliers tend to scale this process through batch reactors, keeping a close eye on temperature and acidity to avoid over-oxidation. Experienced chemists use established purification techniques—recrystallization or column chromatography—to reach high product purity. Insights gained over years show that slight tweaks in reagents or temperature impact yields dramatically—a fact many new chemists learn the hard way when optimizing even “simple” reactions.
In the lab, 2-thiophen-2-ylpropanoic acid offers a versatile handle for numerous reactions. Its carboxyl group allows for classic transformations—amidation, esterification, reduction to the alcohol, conversion to acid chlorides, and even coupling reactions to yield more complex heterocycles. The methylene bridge next to the thiophene brings a degree of reactivity, with α-hydrogen abstraction enabling enolate chemistry and further functionalization. The electronic influence exerted by sulfur within the ring affects electrophilic substitution, a critical consideration for any synthetic chemist planning downstream applications, from medicinal leads to advanced materials.
Scientists refer to this molecule by a range of names, including 2-(2-Thienyl)propanoic acid, 2-(Thiophen-2-yl)propionic acid, or simply its assigned CAS number 2217-07-8. Across supplier catalogs, additional names might pop up depending on international conventions or historic publication references.
Working with 2-thiophen-2-ylpropanoic acid, chemists rely on standard safety approaches found in most organic synthesis labs. The molecule’s sulfur ring and acid group warrant the use of nitrile gloves, chemical goggles, and fume hood precautions. Spills or contact prompt rinsing with copious water, and chronic inhalation risk remains low but not negligible. Waste disposal follows guidelines for sulfur-containing organic materials: segregate, neutralize acids, and use licensed chemical disposal contractors or regional waste management authorities. Over the years, workplace audits have highlighted the importance of accessible safety data sheets and proper labeling, especially where new researchers cycle through academic labs.
This compound turns up in a surprising span of industries. Pharmaceutical researchers have tested it as part of nonsteroidal anti-inflammatory drug (NSAID) discovery, modeling it after known active compounds to seek improvements on metabolic stability or target selectivity. It also acts as a synthetic intermediate for more elaborate heterocycles, which crop up not only in drugs but also in advanced polymer science and organic electronics. Agrochemistry has explored derivatives for their potential as herbicides or growth modulators, owing to the unique influence of the thiophene ring on bioactivity. Even outside these high-visibility sectors, academic projects harness it as a starting block for coursework on modern organic reactions—younger chemists get hands-on experience without the risks of exotic or highly toxic intermediates.
Ongoing research in academic and big-pharma sectors fixates on harnessing this acid for new therapeutic scaffolds. Published studies probe structure–activity relationships by modifying substituents around the thiophene and propanoic acid portions. At major chemistry conferences, presenters recount successes and dead-ends from efforts to develop analogs with improved in vivo profiles or novel mechanisms. Materials scientists explore polymerizable derivatives, betting on sulfur’s influence to tweak electronic properties for use in sensors or thin-film transistors. Compared to a decade ago, computational chemistry now plays a larger part, predicting the effect of various modifications before chemists step into the lab.
Work on the toxicity profile of 2-thiophen-2-ylpropanoic acid sits both in scientific papers and regulatory submissions. Acute toxicity studies in animal models suggest a low to moderate risk, with primary effects at high doses centered on gastrointestinal irritation, consistent with other weak acids. The thiophene ring occasionally raises suspicion given its relation to bioactivation in the liver, so researchers take extra care in chronically dosed models. Metabolism studies pin important oxidation reactions on the sulfur atom, so monitoring for reactive intermediates can’t be ignored. Despite the mild manner of the base compound, regulatory agencies urge clarity on storage, handling, and downstream metabolite clearance, especially if analogs reach late-stage development.
Looking ahead, the future for 2-thiophen-2-ylpropanoic acid shines brightest in pharmaceuticals and advanced materials. Chemists anticipate that targeted modification—be it new substitutions or hybrid structures—will unlock further therapeutic opportunities, especially as drug resistance trends push discovery into new chemical spaces. In materials science, efforts to embed functionalized thiophenes into conductive polymers align with the global chase for better solar cell and sensor performance. As synthetic routes grow both greener and more efficient, production costs start to drop, opening doors to wider commercial adoption. Universities and startups are building on this momentum, driven by the dual promise of new drugs and smarter materials. Whether in a research bench vial or a pilot-plant drum, this compound remains a driver for innovation—one well deserving of its place in the modern chemist’s toolkit.
If you spend enough time around pharmaceutical or chemical research, you’ll see a lot of long, complicated names. 2-Thiophen-2-Ylpropanoic acid isn’t one of the words that most people will recognize, but for those working in labs, it has special meaning. It’s a building block—a way to move forward in drug development and scientific experiments. This compound weaves into the story of molecules that can affect real lives, behind the scenes, away from headlines and TV.
Most folks never encounter 2-Thiophen-2-Ylpropanoic acid in daily life. In research labs though, it’s a small piece of a big puzzle. Scientists use it to produce new molecules for medicine. It’s what chemists call a “precursor,” a bit like an ingredient in a recipe that gives you a new flavor or texture once you mix it in. I’ve seen firsthand how even a slight tweak in a molecule’s structure can take a compound from useless to life-changing. 2-Thiophen-2-Ylpropanoic acid is often the kind of ingredient researchers use to try new things—looking for the next pain reliever, anti-inflammatory, or treatment for a rare disease. These experiments don’t always pay off, but the search never stops.
In practical terms, chemists usually select this compound for its thiophene ring—a component that helps build more complicated chemicals. Drugs containing a thiophene ring have found their way into everyday medicine cabinets, treating asthma, epilepsy, and more. With 2-Thiophen-2-Ylpropanoic acid, researchers often aim to create molecules with specific physical and biological properties. This hands-on experimentation is where science gets interesting. The process is slow. It’s not glamorous. Some days end without a breakthrough. But without people working quietly on fundamental pieces like this one, major progress rarely happens.
In labs, the routine around chemicals stays strict. 2-Thiophen-2-Ylpropanoic acid needs careful storage and handling because safety isn’t just a formality—it’s a necessity. Exposure can bring risks, so the right gear and training matter. These protocols aren’t about checking boxes. They protect people who put in the hours at the bench, juggling curiosity with responsibility. Mistakes can slow down projects or, worse, put someone out of action. I’ve seen more than one talented scientist sidelined by a preventable mishap. Safety practices make progress possible.
There’s another side to the story—getting reliable access to high-purity chemicals without cutting corners. Lab budgets often buckle under the pressure of sourcing quality materials. If researchers skimp on quality, experiments can fail, leading to wasted months or bad data. Strong supplier relationships and clear traceability back to the manufacturer make a real difference. In my experience, investing in reputable sources pays off over the long run, even if it eats into short-term budgets. When corners get cut, the science pays for it in the end.
The best science happens when researchers can trust their tools, from precision instruments down to each chemical on the shelf. 2-Thiophen-2-Ylpropanoic acid serves as a reminder: innovation depends on every step lining up with care. Regulatory frameworks around chemical use in research matter, too—they help weed out unsafe practices and keep the work transparent. Professionals who keep up with current best practices, both in handling and sourcing, are better positioned for breakthroughs. That circles back to trust. Trust in data, trust in safety, and trust in the basic building blocks themselves.
2-Thiophen-2-Ylpropanoic Acid hints at a unique blend of aromaticity and acid functionality. Picture thiophene—a five-membered ring with a sulfur atom—and imagine it attached at the 2-position to the side chain of propanoic acid. Decoding this, you get a benzene-like ring, but with sulfur instead of a carbon, and a three-carbon chain that ends in a carboxyl group. Pretty straightforward for those who’ve drawn skeletal formulas in college organic labs.
Looking at the structure, you count the atoms: The thiophene ring brings four carbons, four hydrogens, and a sulfur. The propanoic acid group gives you three more carbons (two from the chain, one inside the acid group), four more hydrogens, and two oxygens. Putting them all together, 2-Thiophen-2-Ylpropanoic Acid clocks in at C7H8O2S.
The numbers matter beyond just being trivia. Labs, chemists, and the pharmaceutical world rely on formula and weight for everything from ordering supplies to dosing medicines. Let’s talk numbers—its molecular weight stands at 156.20 g/mol. Sizing up the molecule precisely keeps research on track and costs in check.
Organic synthesis, the backbone of industrial and pharmaceutical chemistry, dances with details. The molecular formula answers the simple but vital question: “What exactly am I working with?” One wrong atom in the mix, and a reaction can turn into a mess—a well-stirred batch of nothing useful. Quality control teams run analysis after analysis, always matching the product to the theoretical C7H8O2S target.
Molecular weight drives practical decisions too. Everything from calculating how much to add to the flask to balancing a reaction equation leans on that 156.20 g/mol number. Miss it, and you end up with wasted material and skewed results. It’s not just a line in a database; it’s the baseline for all lab math. I can't count the number of times I’ve recalculated doses or quantities in a lab notebook, just because getting this figure right means the rest of the work matters.
Academic research thrives on precision. Analysts lean heavily on techniques like NMR and mass spectrometry to confirm both the molecular formula and the integrity of a compound. Just because a bottle has the right label doesn’t mean it contains pure material. Contamination and mislabeling still trip up projects. That’s why analytical data go beyond trust—they're about backup. My years in graduate school taught me to double-check—no matter who wrote the label.
Another layer to this is supply chain transparency. A reagent listed as C7H8O2S by a supplier isn’t always a guarantee of quality. Laboratories often struggle with unpredictable purities and delays. A tighter focus on standardized sourcing and sharing spectral data can reduce uncertainty and keep experiments running smoothly. If more suppliers shared third-party test results up front, we'd all waste far less time troubleshooting.
Every research breakthrough starts small, with known, trusted building blocks. The right molecular formula and molecular weight help researchers, students, and industry pros avoid unpleasant surprises. Reliable sourcing, analytical honesty, and attention to detail on the bench keep the wheels of discovery turning—day in, day out. Whether chasing a new medicine or fine-tuning a catalyst, chemistry asks for precision, not perfection.
2-Thiophen-2-Ylpropanoic Acid might sound like a mouthful, but for anyone handling chemicals—whether in a research lab or a chemical supply warehouse—the basics haven’t changed. If you’ve spent any time storing specialty chemicals, you already know each one comes with its own quirks. This compound, built around a thiophene ring, will act up if kept in the wrong environment. A lot of people in academia ask about storage because this acid tends to degrade if ignored.
Every bottle of 2-Thiophen-2-Ylpropanoic Acid I’ve ever seen wears the same warning: keep it cool and dry. Temperature always matters. Heat can transform stable chemicals into something unpredictable. Cool storage—think 2-8°C, which matches the standard for many organics—keeps breakdown at bay. Leaving it on a countertop or near a sunny window? Eventually, you run into color changes, altered pH, or even the scent shifting from typical to sulfur-like staleness. That’s a signal degradation started.
Humidity never does a chemical collection any favors, and organic acids pull moisture out of the air. Water exposure really speeds up hydrolysis and messes with purity. So anyone working with this compound should keep bottles tightly sealed, ideally in glass with chemically resistant caps. I’ve seen plastic stoppers collapse around organic acids over time—particularly the aggressive ones.
Oxygen often takes the blame in storerooms. Oxygen in the air can react with sensitive compounds and kick off unwanted side-effects. With 2-Thiophen-2-Ylpropanoic Acid, people who use inert gas (nitrogen or argon) for blanketing often see a much longer shelf-life. It’s a trick picked up from synthetic chemistry, but anyone can do it if they have the right equipment. I’ve watched colleagues work through cases in poorly capped bottles, and losses can pile up quickly—no one enjoys ordering a replacement for a chemical that broke down just sitting on the shelf.
Sunlight makes matters worse. Many organic compounds change or break down under strong light. The dark brown or amber glass bottles are not just for show. They actually cut down on UV exposure. Store chemicals like this behind a solid cabinet door or away from direct sources of light. One time, a colleague left a bottle in their window for a few weeks, and the entire contents had yellowed. They couldn’t trust it for any reactions afterward.
Many people rush through daily chemical inventory checks. Every so often, I’ve made a habit to look at my stock: labels clear, no crystals forming on caps, no leaks or bulging. If the acid starts to crystallize outside or around the cap, that might mean air sneaked in. Storage in a chemical refrigerator works well, but it matters just as much to have everything dated and rotated.
Don’t transfer to smaller containers unless everything stays sterile and dry. Every extra bottle means an opportunity for air, water, and light. I use a primary glass storage bottle, not plastic, and only pull out what’s needed for the experiment. Anything unused stays capped right away.
2-Thiophen-2-Ylpropanoic Acid carries risks—skin contact burns, inhalation harms. Gloves and goggles ought to stay close by when handling bottles. In case of spill or expired stock, follow hazardous waste rules, bag it, label it, and keep it isolated for disposal. Good chemical hygiene cuts the odds of contamination, helps meet regulatory expectations, and keeps labs running without unnecessary surprises.
2-Thiophen-2-ylpropanoic acid stands out as an aromatic carboxylic acid, shaped around a thiophene ring. In plain language, it has close ties to common organic building blocks found in labs and manufacturing. Many people will never cross paths with this compound outside of a research or industrial environment.
Labwork has always brought some measure of danger, no matter the chemical. Researchers handling 2-thiophen-2-ylpropanoic acid need to stay alert. The thiophene structure resembles compounds in medicines and agricultural agents, but similarity in shape doesn’t mean safety.
No major database, including PubChem and ECHA, lists this acid as outright acutely toxic. That gives researchers a little breathing room, but it does not guarantee safety. It doesn’t show the telltale warning symbols of notoriously hazardous chemicals, like benzene or cyanides. On paper, that means lower risk for acute poisoning or carcinogenic effects—but real-world conditions ask for more caution.
A substance like 2-thiophen-2-ylpropanoic acid can still cause problems if it touches skin or eyes. Chemistry sometimes tricks even seasoned workers; a batch containing a thiophene ring can irritate, especially with repeated spills or splashes. I learned early on in my own experience with similar compounds that gloves and goggles aren’t optional—they’re the last line of defense against accidental burns or rashes.
Breathing in powders or tiny droplets should always be avoided. At times, unfamiliar substances trigger headaches, coughing fits, or more severe respiratory distress. Good ventilation and regular handling lists in the lab limit these risks. No study so far labels this acid as a respiratory sensitizer, but common sense says: why gamble with your lungs?
Disposal means more than pouring solutions down a drain. Many aromatic acids, especially those with sulfur in the mix, can linger in soil or water. Over time, this affects microbes, insects, even fish. My own past experience working with sulfur-based chemicals showed that waste buildup can surprise whole teams and trigger expensive cleanups. Tracking and labeling all containers cuts down on those surprise emergencies.
Spills shift risks from people to wildlife and waterways. Because of this, labs and factories need to use proper disposal channels—dedicated chemical waste bins, incineration under controlled settings, and regular inspections. These steps keep water and soil from picking up contamination no one feels until it’s too late.
Every chemical brings responsibility. I remember one workshop where an old-timer put it simply: “Assume any new powder or liquid can bite, even if nobody’s written it down yet.” Those words stuck with me. Data gaps around long-term exposure, hormonal disruption, or DNA damage do not mean safety—only that no one has looked closely enough, or for long enough.
For now, gloves, eye protection, working under a fume hood, and clear waste disposal stand as the best choices. Manufacturers and lab managers should always run hazard reviews before introducing less-common acids like this one into daily routines. Reviewing MSDS sheets, teaching teams to spot early symptoms, and logging every accident or near miss create a culture of safety.
Chemistry brings progress, but health keeps the work going. That equation applies to 2-thiophen-2-ylpropanoic acid just as much as any other reactive substance.
Dealing with fine chemicals often means obsessing over tiny details. Purity ranks high on that list. With 2-Thiophen-2-Ylpropanoic Acid, most reputable suppliers deliver it at 97% purity or better. That number isn’t random. It reflects the general demand from researchers and pharmaceutical developers who need reliable building blocks for further synthesis. Lower-grade material, loaded with unknown byproducts, can easily grind an entire project to a halt, especially in medicinal chemistry where trace contamination corrupts both results and product safety.
From my own time spent prepping batch reactions, chasing anything below 95% rarely worked out. Separating an active compound from a soup of byproducts takes time and resources few labs want to spare. Having 97% or higher means less time troubleshooting and more focus on actual research. Analytical data supports these choices, and quality control teams look for clean melting points, consistent spectral data, and tight certificate of analysis reports before even thinking about opening the bottle.
Small-scale chemistry doesn’t call for buckets of fine chemicals, so the most common size for 2-Thiophen-2-Ylpropanoic Acid lands in the 1-gram to 5-gram range. This suits early-stage pharmaceutical research, academic syntheses, and method development. Most chemists don’t need more unless it’s time to scale up — nobody wants half-empty bottles turning crusty or soaking up moisture week after week.
Working at a contract lab gave me a real sense of how wasteful “economical” large packs can be. That 25-gram bottle seems cheap until half the powder goes bad waiting on a shelf. Smaller packaging also helps manage risks — if something is found to be out of spec, only a small batch needs disposal, not an expensive kilo. That can save a lot of explaining with quality assurance, not to mention lost time.
For process teams or pilot plant runs, larger bottles or drums get the call: 25 grams, 100 grams, even up to kilograms when a recipe proves its worth. Suppliers tend to offer these bigger lots by special order. Pre-packed bottles help with traceability, and any ordering system worth its salt keeps records tight. The cost jumps, but for established routes or diagnostics, buying bigger gets you consistency from the same lot.
Purity specs and clear packaging aren’t marketing fluff. Supply chain groups reviewing audits want to see certificates, batch numbers, and traceable records. These documents back up everything from basic lab notes to international shipping. Recalls or issues go a lot smoother with those details in hand.
While a bottle may look like any other, what’s inside tells a story: reliable purity promises safer reactions and reproducible data. Genuine labeling and thoughtful sizing keep work safer, smarter, and more cost-effective—right down to the last gram.
I always check paperwork and expiration dates. Some suppliers cut corners, especially on storage or documentation, but it shows up in the paperwork long before it does in your experiment. Reliable sources willingly certify their purity, describe shelf life, and offer sizes that make sense for real projects.
Choosing purity and size for 2-Thiophen-2-Ylpropanoic Acid matters as much as the experiment itself. Both shape the quality of work, the trust in the results, and the track record of any lab that cares about good science.
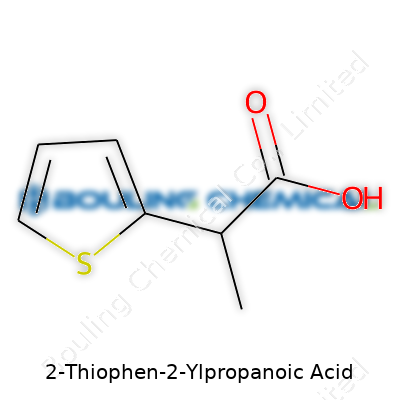
| Names | |
| Preferred IUPAC name | 2-(Thiophen-2-yl)propanoic acid |
| Other names |
2-(2-Thienyl)propanoic acid α-Methyl-2-thiophenylacetic acid 2-Thiophenepropanoic acid |
| Pronunciation | /tuː-θaɪˈoʊfɛn-tuː-ɪl-proʊˈpeɪn.oʊ.ɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | [19139-72-9] |
| Beilstein Reference | 1434976 |
| ChEBI | CHEBI:89618 |
| ChEMBL | CHEMBL286506 |
| ChemSpider | 147680 |
| DrugBank | DB07294 |
| ECHA InfoCard | 03b7c8a7-39a7-493f-82f1-7eea1bed04a9 |
| EC Number | 43036-12-4 |
| Gmelin Reference | 1620924 |
| KEGG | C18702 |
| MeSH | D015633 |
| PubChem CID | 3125782 |
| RTECS number | XN8575000 |
| UNII | 5Q68Z3F0IU |
| UN number | UN3334 |
| Properties | |
| Chemical formula | C7H8O2S |
| Molar mass | 170.22 g/mol |
| Appearance | white to off-white solid |
| Odor | aromatic |
| Density | 1.24 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.9 |
| Vapor pressure | 0.0000136 mmHg at 25°C |
| Acidity (pKa) | 4.1 |
| Basicity (pKb) | pKb = 15.04 |
| Magnetic susceptibility (χ) | -61.07·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5700 |
| Viscosity | Viscous liquid |
| Dipole moment | 1.98 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 226.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -137.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2679.9 kJ/mol |
| Pharmacology | |
| ATC code | N02AX06 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: "P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P312, P321, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 103 °C |
| LD50 (median dose) | LD50: Rat oral 940 mg/kg |
| NIOSH | SKA74 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 20 mg/m3 |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Ibuprofen Flurbiprofen Naproxen Ketoprofen Fenoprofen |