Chemists first encountered 2-Thienylcarbonyl chloride more than half a century ago. The push to make sulfur-containing building blocks for pharmaceuticals, dyes, and agrochemicals sparked demand for new thienyl derivatives. Early organic chemistry pioneers refined ways to chlorinate thiophene carboxylic acids, carving out laboratory routes that balanced yield and practicality. Over decades, efficiency gains and improved safety protocols followed, driven by tight regulatory scrutiny and growing industrial appetite for tailor-made aromatic acids and their acyl chlorides.
This compound stands out as a classical acid chloride capping a five-membered, sulfur-rich thiophene ring at its second position. Its sharp, stinging aroma signals reactivity, not gentleness. Most handlers know it as a colorless to pale yellow liquid, and suppliers usually ship it in amber glass with secure caps to block light and moisture. Any significant batch comes with a purity report, and those needing highest standards often see HPLC or NMR data showing minimal impurities or degradation.
2-Thienylcarbonyl chloride's boiling point hits about 116-118°C at 15 mmHg; its density registers near 1.35 g/cm³. Water reacts with it immediately, so open flasks mean a foul-smelling cloud and wasted material. Volatility stays relatively high for an acyl chloride, and storage conditions matter—keep it cool and dry, never open to air for too long. Chemically, this compound acts as a potent acylating agent. Its thienyl group directs reactivity and offers a unique aromatic bite, unlike benzoyl chloride’s blander character. Lab hands track its corrosiveness—skin burns, vapor stings the eyes and lungs, and copper fittings turn green in its presence.
Labels for any vial or drum spell out much more than just "C5H3ClO2S." Common tags include its molecular weight of 162.6 g/mol, batch purity, and essential storage instructions. Safety icons rarely get skipped: corrosive pictograms, glove and goggle warnings, and recommendations for storing it well away from heat or bases. Most vendors rely on UN identification codes for international shipments, and clear batch traceability helps recall any suspect material at the first hint of contamination or mishap.
The most trusted synthesis pathway starts with 2-thiophenecarboxylic acid. Thionyl chloride delivers the sharpest conversion, with careful control given to stirring rates, moisture exclusion, and side-reaction suppression. Some specialists swap in phosphorus trichloride for gentler conditions or when working with sensitive attached groups. Post-reaction workup always means driving off excess thionyl chloride or phosphorus reagents under reduced pressure, pulling out the product with gentle heat, followed by vacuum distillation for cleanest fractions. Amateur setups face serious risks from exotherms, acid fumes, and fire hazards—experienced chemists never shortcut ventilation and protective setups.
This compound shines as a foundation for making more complex molecules. Organic chemists rely on it to acylate amines, giving rise to 2-thienylamides found in various small molecule drugs and advanced materials. Reaction with alcohols produces thienyl esters, while addition to hydrazines builds new scaffolds for dyes or ligands. The acid chloride function opens countless doors in peptide chemistry: chain extensions and selective protection strategies can run more smoothly than with sterically hindered or sluggish substitutes. Extra chlorines sometimes tag along as impurities, so careful analytical checks ensure clean product for demanding applications.
Scientists refer to this molecule under several titles. Besides "2-Thienylcarbonyl chloride," one often sees "2-thenoyl chloride" in pharmaceutical literature, or "Thiophene-2-carbonyl chloride" in synthetic protocols. In German or French technical catalogs, names shift slightly, yet point unmistakably to the same reactive thienyl system, signaling the same risks and rewards for those exploring organosulfur pathways.
Strict quartets of glove changes, fume hoods, and unblinking eye protection set the ground rules. Splashes can trigger deep skin burns. Inhaled fumes scrape at the lungs. Spill kits never sit far away, and neutralizing agents—often sodium bicarbonate solutions—stand at the ready to quench rogue drops. Disposal never gets casual, since waste streams must pass through neutralization and often strict halogenated organics containment. Full compliance with OSHA, REACH, and local chemical handling rules follows real experience: anyone who’s rushed, skipped gloves, or skimped on ventilation remembers the biting pain or angry red welts for months afterward.
2-Thienylcarbonyl chloride proved itself beyond academic curiosity, carving a real industrial role in drug synthesis and fine chemical production. Medicinal chemists favor its unique combination of aromatic and electron-rich character, which imparts pharmacologically interesting twists to amides, esters, and hydrazides. Agroscientists build on these reactions for pesticides and veterinary agents where sulfur heterocycles outperform plain benzenoids. Material scientists modulate polymers with thienyl motifs for electronics or sensors, and a handful of new OLED and photovoltaic technologies transition from benchtop prototypes to early scale-up batches, all launching from this acid chloride’s foundational reactivity.
Innovation in the lab centers on wringing more selectivity and efficiency from 2-Thienylcarbonyl chloride. Some teams push greener synthesis, swapping traditional chlorination agents for enzymatic or electrochemical upgrades. Others optimize reactivity under milder conditions, limiting waste and reducing toxic byproducts. Collaboration across pharmaceutical teams, agrochemical startups, and academic labs keeps identifying new target molecules with improved biological profiles, using the thienyl motif as a key handle. Researchers continue to publish new crystal structures, process optimizations, and analytical techniques tailored to tracking trace impurities and verifying reproducibility in larger-scale custom synthesis.
Laboratory evidence highlights its acute toxicity on direct contact. Studies in animals show respiratory effects, organ damage, and corrosive injuries at modest doses. Eye irritation and pulmonary distress prompt most regulatory agencies to require strict exposure limits and detailed handling protocols. Skin absorption risk shapes best-practices for gloves and emergency eyewash stations. Most research agrees that environmental persistence remains limited, thanks to hydrolysis, but localized contamination from spills or improper disposal can cause concentrated hot-spots with real risks to aquatic or terrestrial organisms.
With industry demand for more sophisticated sulfur heterocycles trending upward, future prospects for 2-Thienylcarbonyl chloride look solid. New green synthesis pathways may shift production away from hazardous chlorinating agents, aligning more closely with modern sustainability goals. Expanded regulatory acceptance in developing markets for pharmaceuticals and specialty polymers puts merchant manufacturers in a strong position to scale up offerings with stricter quality monitoring. Ongoing research keeps discovering fresh bioactive targets and functional materials woven around the unique chemistry of the thienylcarbonyl group. Young chemists picking up the flask see plenty of room for discovery, a clear opportunity to build on past experience and shape more responsible, versatile chemistry for years ahead.
2-Thienylcarbonyl chloride might sound complicated, but its formula boils down to C5H3ClOS. This compound holds a special place in organic chemistry because it marries the structure of thiophene, which is a five-membered ring with a sulfur atom, with the reactive acyl chloride group. In the lab, C5H3ClOS does more than just sit on a shelf; it works as a powerful building block for making other complex molecules.
The backbone of 2-Thienylcarbonyl chloride comes from thiophene. Picture this ring—four carbon atoms plus one sulfur atom—stable and aromatic. Stick a carbonyl chloride group at position two on this ring, and you get a molecule that’s both stable and reactive. Putting these pieces together, chemists write it out as C5H3ClOS, showing five carbons, three hydrogens, one chlorine, one oxygen, and a sulfur atom rounding out the team.
Back in my postgraduate years spent in synthetic labs, reagents like 2-Thienylcarbonyl chloride helped crank out all sorts of pharmaceuticals and advanced materials. Knowing its formula—C5H3ClOS—didn’t just satisfy a test question, it meant understanding exactly what would happen in a flask, how it might react with sensitive amines or alcohols, and what byproducts could roll out. Nobody wants a halogenated mess ruining an expensive batch of product. Precision isn’t just for theory. In one run, a confusion with structural isomers almost shut down a project for a week. Chemistry rewards those who pay attention to details like formulas.
Making 2-Thienylcarbonyl chloride takes skill. It usually comes from reacting 2-thiophenecarboxylic acid with reagents like thionyl chloride. The process throws harsh fumes and corrosive vapors into the air. Fume hoods see heavy duty, and we rarely skipped over personal protective equipment. In my early days, a careless glove disposal led to residue burns—a reminder that even small oversights with reactive chemicals carry real risk.
C5H3ClOS gets into the action when chemists look to attach a 2-thienyl group to larger pharmaceutical frameworks. It gives researchers a reliable tool for making heterocyclic derivatives—essential cores for drugs targeting everything from infections to neurological disorders. Industry sees it as more than a tool; it’s almost a passport to more effective medicines and advanced materials.
Compounds with the acyl chloride group, especially ones containing a sulfur atom, deserve proper respect outside the lab too. Waste needs careful neutralization. Discharging leftover material without treatment threatens water and soil, and as someone who grew up near a manufacturing site, I saw first-hand what chemical mishandling can do to local wells and wildlife.
Laws continue to tighten around hazardous chemicals, pressing scientists and companies to rethink their waste streams and exposure risks. Training has grown stronger, and electronic tracking connects batches of C5H3ClOS to every stage of research and production. Open conversations about risk aren’t just encouraged—they’re essential for keeping people and the environment safer. My own experience has shown that a simple review before every batch pays huge dividends—catching minor errors before they become problems and protecting everyone in the process.
Every chemist who’s spent time in a research laboratory can spot a flask of 2-Thienylcarbonyl chloride by its pungent odor alone. This molecule shows up in research catalogs as a useful building block, but that barely scratches the surface. It pops up in so many pharmaceutical and agrochemical projects because it brings a handy combination of reactivity and structure. The thienyl ring offers something different than the typical benzene derivatives—sometimes, that one switch means a compound can jump from failure to promising candidate.
You’ll find this molecule most often behind the scenes of drug discovery. It steps in as a coupling partner for making thienyl-based amides, hydrazides, and ureas. Chemists use it to add the thienyl group, aiming for enhanced biological activity or better selectivity in drug candidates. For example, the presence of a sulfur atom in the thienyl ring can encourage specific interactions with biological targets, leading to molecules that act differently than plain phenyl variants.
In my own graduate lab years, we looked for small tweaks that could improve both activity and metabolic stability. Swapping a benzoyl for a thienylcarbonyl moiety sometimes unlocked new antibacterial or antifungal properties. A few published papers trace their roots right back to that decision—an easy reaction with an amine or hydrazine, followed by biological screening. The fact that so many pharmaceutical patents mention this compound as a key synthetic intermediate shows its staying power.
Not only drug companies reach for 2-Thienylcarbonyl chloride. Agrochemical researchers use it when developing new crop protection agents, like herbicides or fungicides. The thienyl structure helps modify the interaction of these chemicals with enzymes in weeds or fungi. Some commercial products protecting fields today started their journey with a batch of this stuff bubbling away in a hood.
Agriculture moves quickly, and pests adapt even faster, so scientists are constantly changing the recipe. A tweak to the molecular handle—a thienyl instead of a benzoyl—sometimes dodges resistance or increases uptake in plants. That sort of change can keep food supplies more secure and cash crops growing strong, especially as global challenges mount.
Move beyond health and crops, and you’ll see 2-Thienylcarbonyl chloride used for custom dyes, advanced polymers, and organic electronics. Its ability to couple with a variety of nucleophiles (amines, alcohols, hydrazines) gives synthetic chemists options. Certain specialty dyes and pigments for textiles and imaging rely on thienyl structures to achieve unique optical properties or stability. The world of OLED displays sometimes leverages the electron-rich thienyl ring for light emission and conductivity, and this acid chloride has played a part here too.
On shop floors and in small research startups, the decision to use 2-Thienylcarbonyl chloride usually comes down to performance. Scientists want colors that don’t fade, plastics that bend without breaking, or sensors that pick up faint signals. Its role in these materials connects chemistry from the backroom lab bench straight to what you see and use every day.
It’s impossible to avoid discussion of safety when dealing with acid chlorides like this one. Every chemist has a story about that sharp, eye-stinging vapor. Labs need good ventilation, careful glove use, and storage away from water. Researchers keep pushing for greener alternatives and milder reaction conditions. Solvent choices and waste reduction matter more now, with increasing regulatory and public attention. Working with this compound in a responsible way supports both the health of lab workers and the long-term reputation of the chemical industry.
Working with 2-thienylcarbonyl chloride in the lab means spending time with a reactive, corrosive chemical that won’t wait for you to make a mistake before causing harm. It fumes in humid air, attacks eyes and skin on contact, irritates the lungs, and adds an extra layer of danger by releasing hydrogen chloride vapor and fumes when spilled or heated. Chemists who use this substance know that being casual with the bottle can bring not just pain, but also long-term health problems. I’ve handled similar acyl chlorides, and learned quickly that a careless moment can mean a hospital trip.
No splash goggles, no experiment. Regular safety glasses do nothing if the fumes hit your eyes, and these chemicals can burn before you realize what has happened. Choose goggles that seal tight, not just standard fare, and step up to a face shield for transfers or larger reactions. Skip the latex gloves—go straight for heavy-duty nitrile or neoprene, because the wrong gloves melt fast. Old lab coats won’t keep you safe from splashes; acid-resistant coats and sleeves cover exposed skin and keep unexpected leaks from soaking through. Wearing long pants and closed shoes is part of the basic dress code, but treating it as a suggestion often ends as a story shared in safety trainings.
Even small amounts of vapor from a thienylcarbonyl chloride solution sting the throat and make you cough. Air must flow, and that means running all reactions, weighing, or transfers inside a well-functioning chemical fume hood. Always keep containers in the back of the hood, maximizing distance from your face. High-efficiency ventilation stops fumes from wafting out into your space or down the hall. If your hood alarm sounds or airflow drops, don’t risk it. Wait for repairs before continuing—no shortcut is worth a lungful of acid.
Store thienylcarbonyl chloride in a tightly sealed bottle, in an acid-cabinet made for strong corrosives, nowhere near water-reactive or strong base chemicals. Mixing these by accident leads to messes and panic. I’ve seen new students put reactive bottles on open shelves—one tipped container or condensation and you’re dealing with fumes all over the lab. Keep the bottle dry, upright, and always labeled. If transporting, use a secondary container or a Nalgene carrier for backup against drops.
Spills don’t give you time to read a manual. Have a spill kit ready before starting and know where it sits, not just in theory. Neutralizers for acid spills, plenty of absorbent pads, and instructions within arm’s reach help keep accidents small. If it hits skin, rinse with cool running water—never just a dab with a wipe. Eyes need a full flush at the eye wash, no pauses, then get to medical help immediately. Vapors reaching the lungs can trigger coughing, burning, and more; leave the area fast and seek help without waiting to see if symptoms get worse.
Training shapes habits. Refresher courses in chemical hygiene, regular safety drills, and honest conversations about near-misses turn caution into muscle memory. Nobody walks into a lab aiming to get burned or gassed, but skipping the basics out of confidence or rush pays a heavy price. I’ve learned from watching others’ mistakes and sharing my own. In chemical work, safety happens by design, not by chance.
Handling 2-thienylcarbonyl chloride safely takes more than gear. It builds from habits, planning, focused teamwork, and respect for chemical hazards. You can learn its uses, but you only stay safe by staying thoughtful each time you reach for the bottle.
Working with chemicals like 2-thienylcarbonyl chloride brings up real concerns in the lab. It's not just a matter of maintaining product quality, but also keeping people out of harm’s way. Many folks look at storage as a minor step, but if you’ve ever cracked open a bottle that released a sharp smell or noticed weird discoloration, you quickly realize things can get dangerous in a hurry.
2-Thienylcarbonyl chloride reacts with water, giving off toxic fumes. It can also corrode skin and eyes if spilled or splashed. A single whiff tells you all you need about whether your lab is safe. Health authorities like OSHA point out how these chemicals attack the respiratory system and require strict handling. In my own experience, I’ve seen improper storage lead to minor disasters: ruined batches, ruined glassware, costly evacuations, and headaches nobody needs.
A tightly sealed glass container works best for 2-thienylcarbonyl chloride. I’ve seen polyethylene containers degrade with time, letting in enough moisture to kick off an unpleasant reaction. Use amber glass if you can—light degrades many organochlorides, and while some people forget, storing it in the dark prevents extra headaches later.
Always keep the container in a cool, dry spot, away from direct sunlight and sources of heat. Moisture acts as an invitation for hydrolysis, and you really don’t want hydrogen chloride gas leaking out. I’ve learned to rely on dedicated desiccators packed with silica gel or another drying agent, rather than general chemical cabinets, since a small leak in humidity control can mean a ruined supply.
I keep any reactive acids, bases or water-sensitive reagents separated from 2-thienylcarbonyl chloride. In one lab, a spilled base came in contact with a forgotten bottle and set off an immediate, nasty odor. It took hours to scrub out. Keeping labels clear and logs up to date lowers those risks. Mixing up containers after a long day is too easy without them.
Even the best storage won’t prevent accidents if people get careless. I grab gloves, goggles, and a lab coat every time. It’s not just about checking boxes; I’ve seen coworkers skip protection “just for a quick pour” and regret it instantly. Stains on lab benches and pitting on metal surfaces stand as reminders of small slips that can get out of hand.
Accidents happen. Keep spill kits and neutralizing agents on hand. My practice is to lock up incompatible reactants separately and run regular training so everyone knows what to do if something leaks or spills. Dispose of waste by using a sealed glass waste container, adding a drying agent to soak up any leftover moisture, and passing it to licensed waste handlers. Department of Transportation rules back up the point: handle and transport only in properly labeled, corrosion-resistant packages.
Working safely with chemicals like 2-thienylcarbonyl chloride takes more than following basic instructions. Each detail in storage matters, from your choice of bottle to your reaction to a small spill. With so much at stake—both for health and research—a careful approach turns routine lab work into something reliably safe.
I’ve spent long hours in labs, leafing through countless data sheets, and I know one thing: when a bottle of 2-Thienylcarbonyl Chloride lands on a chemist’s bench, everyone eyes the spec label before anything else. On paper, folks talk about “purity” like it’s a simple tick-box, but small differences carry plenty of weight once the reactions start bubbling and every decimal matters. Most sources supply this compound with a purity not lower than 98%. A sample with less? I hesitate, especially for medicinal chemistry. The reason is simple: the by-products, even those in trace amounts, could scuttle weeks of planning, throw off product yields, or create unpredictable safety risks.
The biggest enemy here remains contamination with thienylcarboxylic acids and traces of thienyl alcohols. If these sneak into the product, trouble follows. I’ve seen a single patch of impurities drag a reaction off course. In some cases, residual thionyl chloride from manufacture hangs around, capable of reacting with solvents or even open air, causing violent decompositions. Labs that monitor for less than 1% of these stray chemicals safeguard workers, data, and budgets.
A serious supplier should back each bottle with a Certificate of Analysis, listing exact percentages of the main substance and major contaminants. Most reputable sources go further, running checks for water content and sometimes even color index. If you ever open a container and spot a smoky tinge or pungent odor stronger than usual, it signals possible breakdown—always worth a call to the supplier.
Industrial buyers buying in kilos care about these specs for reasons that go beyond chemistry. I talked to a friend in agrochemicals who mentioned how even a half-percent impurity could taint a whole production batch. That means tons of waste or product recalls—big money, but also public trust. In pharma, the bar climbs higher. For a potential drug precursor, the risk runs double: regulatory setbacks and health dangers if testing misses a rogue impurity. Manufacturers invest heavily in process chromatography and validated analytical methods to keep levels in check.
It’s not all doom and gloom, though. Reliable purification can transform rough batches into solid ones. I’ve seen success using fractional distillation under low pressure or careful recrystallization procedures. Still, no purification should start without solid, transparent batch data. Drying over activated molecular sieves sometimes works for water removal, but always under a fume hood—chlorides and water create nasty combinations.
Look at the spec sheet. If it skips impurity percentages, push for details before signing off. A good supplier answers quickly and provides past analytical reports. For buyers with smaller needs—university labs, pilot projects—never assume the sample shipped matches what you ordered last year. Batches shift. Storage matters too; a dark-brown dark-glass bottle and a cool shelf can ward off breakdown. If the label only says “purity >98%” but gives no hint about side products or handling, never be afraid to ask questions. Good chemistry requires more than basic numbers—transparency earns trust.
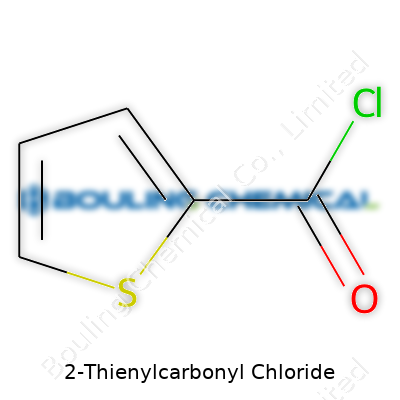
| Names | |
| Preferred IUPAC name | Thiophene-2-carbonyl chloride |
| Other names |
Thiophene-2-carbonyl chloride 2-Thiophenecarbonyl chloride 2-Thienylcarbonyl chloride Thiophen-2-ylcarbonyl chloride |
| Pronunciation | /tuː ˈθaɪ.nɪl ˈkɑː.bə.nɪl ˈklɔː.raɪd/ |
| Identifiers | |
| CAS Number | 4567-24-6 |
| 3D model (JSmol) | `/iviumg9j7qyv5kC9pGL9eDkrRL5zQ9UZ0wLkGgqFp3z75Qfr/8L2Bq93cR4YYn` |
| Beilstein Reference | 1209775 |
| ChEBI | CHEBI:51545 |
| ChEMBL | CHEMBL133420 |
| ChemSpider | 2078886 |
| DrugBank | DB07903 |
| ECHA InfoCard | 01-2119447895-23-0000 |
| EC Number | 2083-12-5 |
| Gmelin Reference | Gmelin 104140 |
| KEGG | C14368 |
| MeSH | D013948 |
| PubChem CID | 69974 |
| RTECS number | XN8575000 |
| UNII | 1A72M7291E |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | DV6Y6WXF43 |
| Properties | |
| Chemical formula | C5H3ClOS |
| Molar mass | 162.63 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | Pungent |
| Density | 1.412 g/mL at 25 °C |
| Solubility in water | Decomposes |
| log P | 1.93 |
| Vapor pressure | 0.7 mmHg (20°C) |
| Acidity (pKa) | 1.70 |
| Basicity (pKb) | Basicity (pKb): 12.31 |
| Magnetic susceptibility (χ) | -45.6e-6 cm³/mol |
| Refractive index (nD) | 1.601 |
| Viscosity | 0.987 cP (20°C) |
| Dipole moment | 3.44 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 268.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -79.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -454.8 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H314, H317, H334, H335 |
| Precautionary statements | P261, P264, P271, P280, P301+P312, P305+P351+P338, P304+P340, P308+P311, P330, P337+P313, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-Thienylcarbonyl Chloride: 3-2-2-W |
| Flash point | 71°C |
| Autoignition temperature | 410 °C |
| Lethal dose or concentration | LD50 oral rat 640 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 472 mg/kg |
| NIOSH | GV5950000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | to be stored at 2-8°C |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Thiophene Thiophene-2-carboxylic acid Thiophene-2-carboxamide 2-Bromothiophene 2-Thiophenecarboxaldehyde |