The journey of 2-Thienylacetyl chloride reflects a strong curiosity in the world of aromatic chemistry, which originally surged in the 20th century as researchers probed the structure of heterocycles. Thiophene, the parent compound, made a splash in the 19th century thanks to its isolation from coal tar and its unusual stability compared to other sulfur-containing rings. Progress in organochlorine synthesis after World War II drove innovation in producing various substituted acyl chlorides. These developments supported pharmaceutical advances, with industry and academia focusing on 2-thienyl derivatives that offered unique chemical versatility. Over decades, chemists have realized the power of thienyl-based acyl chlorides for building blocks in drug discovery, making 2-Thienylacetyl chloride a useful compound for many research labs and commercial operations around the globe.
2-Thienylacetyl chloride shows up as a valuable intermediate for chemists, especially in the synthesis of biologically active compounds, functional dyes, and materials science. Its role as an acylating agent fits perfectly with modern synthetic strategies requiring robust introduction of thienyl functionality. In my own lab experience, this compound shortens the path to specific thienyl ketones, which are central to anti-inflammatory drug design and agricultural chemical development. Most research suppliers offer the compound as a colorless to pale yellow liquid, shipped in airtight glass to avoid hydrolysis. In real-world terms, many researchers reach for this thienyl acetyl chloride when aiming to add diversity to aromatic cores in their pipelines.
2-Thienylacetyl chloride presents as a pungent liquid, typically boiling between 124°C and 127°C at atmospheric pressure. The density lands at around 1.25 g/cm³, making it a bit heavier than plain water. Its chemical fingerprint holds an acetyl chloride functional group attached to a 2-thienyl ring, which sets the stage for high reactivity in nucleophilic substitution and Friedel–Crafts-type reactions. This liquid resists mixing with water, and its color can shift over time due to slow decomposition if left exposed to air or light, producing thienylacetic acid and hydrochloric acid as byproducts. Its reactivity means storage in cool, dark environments is not just recommended — it’s essential if one wants to keep it stable for longer projects.
Quality suppliers supply 2-Thienylacetyl chloride with clear labeling, including molecular formula C6H5ClOS, CAS number 39098-97-0, and batch-specific data like purity (usually above 97%). Labels spell out hazard pictograms—flame for flammability, corrosion for its destructive effects on skin, and exclamation marks for irritancy risk. Some companies even share GC or NMR chromatograms if researchers need top-level confidence for sensitive reactions. During ordering and handling in the lab, this labeling proves crucial, sorting out legitimate reagent bottles from leftover stock that could contain dangerous breakdown products. In regulated industries, correct labeling keeps operators safe and auditors content, reducing risk for both people and companies.
2-Thienylacetyl chloride preparation generally involves reacting 2-thienylacetic acid with thionyl chloride or oxalyl chloride. Many chemists operate under dry argon or nitrogen to keep out water, since even the slightest leak causes unwanted hydrolysis of the acyl chloride group. The exothermic reaction produces hydrogen chloride gas, so well-ventilated fume hoods are a must. After the bubbling fades, excess thionyl chloride and side products like sulfur dioxide get removed by distillation, leaving behind the raw acyl chloride, often pure enough for further use after vacuum filtration. On an industrial scale, manufacturers scale up these methods with better solvent handling and containment. As someone who has made this compound for small-scale projects, attention to dryness and temperature pays off in yield and purity, reducing the headaches that hamper downstream chemistry.
2-Thienylacetyl chloride shines when introduced to nucleophilic partners—primary or secondary amines, alcohols, and water. The reaction with amines yields 2-thienylacetamides, found in a variety of pharmaceutical motifs, and easy transformation to thienylacetamides fuels research in anti-inflammatory and anti-microbial agents. Getting it to react with phenols or alcohols produces esters that play a role as intermediates in dye chemistry. In my hands, I saw the value of this reactivity when adding it to a library of aromatic amines, producing a suite of related products ready for biological screening. For Friedel–Crafts acylations, the 2-thienylacetyl group brings extra conjugation into target molecules, proving useful for both polymer development and specialty electronics. Modifications of the molecule often focus on the thienyl ring, which tolerates further substitution at other positions, multiplying its application in diversified chemical libraries.
On the market and in literature, 2-Thienylacetyl chloride can hide behind several other names: it’s often called Thiophen-2-ylacetyl chloride and 2-[(Chlorocarbonyl)methyl]thiophene. Commercial catalogs highlight its CAS number 39098-97-0 for sure-footed ordering. In some pharmaceutical patents and European chemical indexes, similar spellings and language translations pop up, but all refer back to the same versatile compound. In international journal articles, knowing these synonyms solves plenty of headaches when searching for past research or comparing product specifications, preventing accidental confusion with close structural relatives.
This compound demands strict respect in the lab. It causes burns, damages mucous membranes, and emits choking fumes of hydrogen chloride and sulfur oxides if spilled or overheated. In my experience, double-gloving and using thick nitrile or butyl gloves make it easier to avoid accidental skin contact. Every fume hood session starts with goggles, full lab coats, and vapor-absorbing filters. Spills require neutralization with sodium bicarbonate and ample ventilation. Disposal of waste and glassware requires neutralization and following local chemical hazard guidelines, never pouring down the drain or tossing into general waste. On the safety documentation side, all stocks need up-to-date safety data sheets (SDS), and everyone in a shared lab must know both the risks and emergency protocols. Here, taking shortcuts or skipping steps has brought regrettable accidents to researchers from beginners to old hands.
Research laboratories rely on 2-Thienylacetyl chloride for creating heterocyclic derivatives in pharmaceutical pipelines. Drug designers put it to work building lead candidates for neurological and metabolic diseases, exploiting the thienyl group’s bioisosteric nature as a benzene stand-in. In my field, it played a key role in creating anti-inflammatory scaffolds with improved solubility and metabolic profiles compared to classic benzyl analogs. Agrochemical researchers use it to build up herbicides and insecticides, while in materials science, it helps generate specialty polymers for sensors and organic photovoltaics. The compound supports the synthesis of dyes and intermediates that bring intense, stable hues to electronic and optical materials.
Academic groups use 2-Thienylacetyl chloride to explore new reaction methods, diversify small molecule libraries, and test emerging catalytic processes. Medicinal chemists study its use in fragment-based drug design, given its compatibility with a wide range of nucleophiles. Collaborations between university chemists and commercial enterprises have centered on improving yield and selectivity in acylation reactions, cutting down on byproducts and waste. Emerging green chemistry efforts aim to reduce or replace traditional chlorinating reagents, investigating enzymatic or solid-supported transformation routes. In practical R&D, this focus aims to lower both environmental and economic costs, pushing the field to rethink standard operating procedures.
Scientists have studied the acute toxicity of 2-Thienylacetyl chloride in rodents, finding that exposure causes strong irritation and inflammation along the respiratory tract, skin, and eyes. Chronic exposure remains less documented, but related acyl chlorides tend to generate hydrochloric acid and thienyl derivatives, many of which carry additional risks. In my view, reliable gloves, closed systems, and prompt spill cleanup make an enormous difference in reducing researcher exposure. Experimental data highlights the importance of limiting airborne concentrations—the gas-phase hydrolysis products can lead to corrosive injury if allowed to build up in confined spaces. The compound does not build up in the body, so good lab hygiene and air handling cut the risk to reasonable levels. Long-term environmental impact studies look at its breakdown and effect on aquatic systems, underlining the need for careful waste treatment.
Looking forward, 2-Thienylacetyl chloride will stay in demand in both classic heterocycle synthesis and new pharmaceutical research. Growing interest in sulfur-containing rings for antimicrobial and neurological drugs ensures continued use, especially as companies seek alternatives to benzene-centric pharmacophores. Advances in automation and microfluidic platforms promise safer, smaller-scale synthesis with reduced reagent consumption. Greener preparation routes and biodegradable solvents could further reduce risk to both researchers and the environment. As regulatory agencies tighten restrictions around hazardous chlorinating agents, companies and universities face increasing pressure to innovate both process and product, sustaining the value of 2-Thienylacetyl chloride in tomorrow’s chemical toolkit.
People in labs often reach for 2-Thienylacetyl Chloride during synthesis work. Its chemical formula, C6H5ClOS, isn’t just a string of letters and numbers—each element shapes the reactivity and purpose behind this compound. You can picture the molecule with a thienyl ring (that’s where sulfur comes in) attached to an acetyl chloride group. That little chloride group is what brings reactivity, letting it pair with a wide range of nucleophiles or anchor itself to different chemical frameworks.
Folks in pharmaceuticals and agrochemicals look for molecules that build products efficiently. 2-Thienylacetyl Chloride plays a role as a building block for more complex molecules. Whether it’s helping create new potential drug candidates or specialty chemicals for crops, having a versatile reagent like this saves researchers time. I’ve watched a bench chemist transform a basic starting material using this chloride, speeding up reaction times and opening up new routes for making target molecules.
Why care about the chemical formula in the first place? Reactions depend on understanding these atomic arrangements. Missing a detail on the number of hydrogens or thinking a ring system contains oxygen instead of sulfur can turn a planned synthesis into a messy failure. It’s not just trivia—the details in C6H5ClOS make or break the pathway for what might become the next important medicine or material.
No one picks up an acyl chloride without thinking about safety. From whiffs of irritant fumes to reactivity with water, these aren’t things to ignore. Accidents come fast in the wrong conditions. Even in a classroom demo, I’ve seen professors step in to stress personal protective equipment as soon as the topic comes up, even before discussing the chemical’s applications. The right knowledge and gear matter just as much as knowing the formula. Quick access to fume hoods and spill kits reduces risks. Regular training on handling corrosive reagents keeps people safer and reactions more predictable.
Safety and environmental impact shape the future of chemical work. Labs choosing greener solvents or planning careful disposal after using chloride reagents are already changing the field. Substitution with less hazardous reagents when practical can lower risks for everyone. Digitally tracking chemical inventories cuts down on accidental over-ordering, reducing waste and storage hazards. Sharing lab experiences with reaction outcomes and near-misses can help others dodge expensive or dangerous mistakes.
Every detail about compounds like 2-Thienylacetyl Chloride needs to stand up to scrutiny. Chemists expect to verify a formula with peer-reviewed sources and share reliable data. Being transparent about testing methods, reporting outcomes accurately, and acknowledging failed experiments fosters better science. If a mistake happens, owning up to it keeps the field honest. Clarity and sharing these truths, whether about a successful reaction or an unexpected hazard, inform the next decision someone else will make with the same molecule.
Knowing the formula C6H5ClOS is just the start. What matters more is using that knowledge responsibly to achieve safer, more effective, and innovative chemistry. This is where technical details spark new possibilities.
I’ve worked in chemistry labs where you need more than patience: you need chemicals that open doors to new compounds. 2-Thienylacetyl chloride stands out as one of those problem-solving reagents. This compound’s main draw comes from its active acetyl chloride group tied to a thiophene ring, which gives it unique abilities in the synthesis of organic molecules. Most chemists remember it as an intermediate — a building block that helps you create something more complex, not because of its function by itself, but because of what you can do with it.
Pharmaceutical research often leans on molecules like 2-Thienylacetyl chloride. Drug makers use it for introducing the thienyl group into candidate molecules. This step often improves the biological activity of new drugs. Chemical scaffolding is a big deal in pharmaceutical design. For instance, some anti-inflammatory or anticonvulsant drugs use thiophene rings for better binding in the body. A study published in Journal of Medicinal Chemistry found that adding thiophene rings to molecules shifted their properties in ways that helped absorption and stability. Drug designers often swap out a benzene ring for a thiophene group using reagents like this one, and soon you’ve got novel drug candidates that behave differently.
Companies working on crop protection and pest control chemicals also rely on 2-Thienylacetyl chloride. Some herbicides and fungicides tap into the unique bioactivity that the thienyl structure brings. In my own work with agrochemical startups, chemists keep an eye out for new intermediates with sulfur content. These may fend off pests while leaving crops unharmed. Many patents filed for new agrochemicals list 2-Thienylacetyl chloride among their early key ingredients, chosen because the resulting molecules often fight fungal threats without triggering resistance so quickly.
Material scientists like this chloride for its ability to link up organic molecules in new ways. Thiophene-based compounds can form the backbone for organic electronic materials — think of components in OLED screens, semiconductors, and solar cells. Some advanced dyes and pigments also trace their origins back to reactions involving 2-Thienylacetyl chloride. The sulfur atom in the thiophene ring adjusts the color and stability of these synthetic dyes, leading to deeper colors and improved performance outdoors.
Handling 2-Thienylacetyl chloride calls for some respect. The chemical’s reactive nature means chemists must avoid breathing it in or letting it touch skin. Labs invest in good ventilation and personal protective equipment to work with it safely. Reports of accidents serve as steady reminders: missteps can lead to dangerous releases of hydrogen chloride gas. Regulatory agencies like OSHA keep tight controls on this category of chemicals, especially where large-scale synthesis takes place.
Some of the environmental impact tied to 2-Thienylacetyl chloride can be reduced. Green chemistry initiatives look at ways to make and use such reagents with less waste and safer byproducts. Academic groups have tested alternative routes for making similar building blocks with catalytic processes or safer reagents, but the compound continues to have a strong foothold because few drop-in replacements exist yet for its unique combination of reactivity and selectivity.
Chemists need both reliability and flexibility, and that’s what 2-Thienylacetyl chloride brings to the table. It supports breakthroughs across pharmaceuticals, agrochemicals, advanced materials, and dyes. As the industry shifts toward safer practices and greener methods, the uses of such building blocks won’t disappear. Instead, labs will keep searching for smarter ways to work with them or design new alternatives that keep science moving forward — and workers safe.
Let me tell you, nothing wakes you up during a research shift quite like the sharp, nose-burning smell of thienyl-based chlorides. Years in the lab taught me that 2-Thienylacetyl Chloride stands out as both a helpful building block for pharmaceuticals and an accident waiting to happen if overlooked. People hear “reactive acyl chloride” and sometimes shrug it off—until a drop eats straight through nitrile gloves or stings their lungs.
My first mistake with this compound was trusting a flimsy plastic bottle. It warped within weeks, and a tiny leak meant a cleaning headache for days. Everyone working with thienylacetyl chloride deserves better. Glass containers with tight Teflon-lined screw caps provide real security. Label everything clearly with date, hazard, and your initials. No one needs “mystery bottles.”
A busy bench often obscures the simplest fix. Moisture transforms 2-Thienylacetyl Chloride into a hissing, corrosive mess, and sunlight degrades it quicker than most would expect. The safest place is a desiccator stored in the chemical fridge, well away from peroxides, acids, or amines. Temperature swings can make even capped bottles develop pressure, risking bursts or leaks. On that chilly top shelf or in a light-proof container, the risk drops.
Too many wonder if disposable gloves are enough. Puddles of 2-Thienylacetyl Chloride burn skin in moments. I switched to thick butyl rubber gloves after one spill left my finger tingling for days. Full goggles and a lab coat with sleeves always buttoned—no excuses. The vapor stings the nose and eyes, and lung damage creeps up quick. Just ask anyone caught by a sudden gust in a poorly ventilated room. Never work outside a fume hood.
Disposing of reactive acyl chlorides in a normal waste bottle led to a terrifying fizzing mess not so long ago. Hydrolysis by accident, and the bottle nearly burst. Sodium bicarbonate quenches the excess, turning it into a less harmful salt and saving headaches. Trained staff and good records keep waste streams separate—an absolute must. Skip the shortcuts here, or the clean-up becomes a science experiment of its own.
Someone fresh in the lab often needs a real walk-through, not just a printed safety sheet. The best labs run training sessions with hands-on examples and direct supervision. Near misses get shared in meetings, not hidden, because one person’s lucky escape informs the rest of the group. If the panic shower’s blocked or the eyewash station sits hidden behind boxes, accidents become disasters.
What counts in real handling is not cheap fixes or wishful thinking, but a shared respect for both the usefulness and risk of these reagents. You’ll likely never see the day a small bit of planning prevents a major injury, though you might hear stories from veterans who did. Chemical safety sticks around longest where people genuinely look out for each other, beyond what a checklist covers.
2-Thienylacetyl chloride sits high on the list of chemicals that demand respect from anyone working in the lab or the plant. Looking at its molecular structure, the “chloride” part warns us right away: risk rides with reactivity. This material releases corrosive hydrogen chloride gas when it meets water, and it reacts harshly with skin and eyes. Breathing its vapors burns airways. So, those working with it can’t afford to grow careless.
Every person who’s handled 2-Thienylacetyl chloride for research or manufacturing can tell stories about how a few seconds of exposure leave red skin, runny eyes, or much worse. Double glove with nitrile or butyl gloves and swap out damaged ones right away. Splash goggles always beat regular safety glasses. Face shields cover what goggles leave exposed. Tyvek gowns, aprons, or full lab coats keep street clothes chemical-free and make it easier to shed layers in case of a spill. Inhalation hazard stays high, so workers use fume hoods for even tiny amounts — nobody sniffs this stuff on purpose.
Dry, cool, and well-ventilated rooms block most surprises. Suppose a bottle sits under sunlight or close to heat: the risk of gas leaking or pressure building climbs fast. Airtight containers, fitted with the right labels (corrosive, harmful) cut confusion. Sometimes the smallest error comes during transfer between bottles. Rushing through that step has led to most of the splash injuries I’ve heard about. Setting up a secondary container or working over spill trays keeps spills from spreading. In odd cases, give the bottle a test chill with ice to keep fumes down — never use water, which may worsen the reaction if anything spills.
If someone splashes 2-Thienylacetyl chloride onto skin, you move fast: rinse with running water for fifteen minutes, remove contaminated clothing, and call medical help. If eyes contact this chemical, the same rule holds — no shortcuts. The chemical can stick to eye surfaces and cause lasting damage. Be ready to flush at eyewash stations immediately. Each person onsite should know how and when to use emergency showers and eyewash units. Those who've suffered fume inhalation need fresh air and medical attention right away, as throat and lung irritation escalates quickly.
Spills aren’t a matter of “if”, but “when” for most labs. I’ve watched teams lose valuable time looking for spill kits stored too far from where risk actually happens. Spill kits with inert absorbents such as vermiculite or sand, plus neutralizers, should sit beside — not across the room from — likely spill points. No water for cleaning, only dedicated neutralizers or dry absorbents. Dispose of waste in chemical-resistant containers, not standard trash.
Even veterans need annual refreshers and new workers need direct supervision. Training covers more than textbook safety: it includes stories, real examples, and mistakes. The best-run operations quiz their teams, check labels often, and run drills on using emergency systems. Having a clear incident reporting system flags recurring hazards before they grow into accidents. Safety grows out of daily habits: correct storage, proper labeling, and quick communication. Organizations that schedule unannounced safety audits often uncover small slip-ups that paperwork alone would miss.
Substitute less hazardous chemicals if experimental goals allow. Automated dispensing equipment reduces splash risk. Upgrading to ventilated chemical storage cabinets adds another layer of security. Investing in better PPE or faster fume sensors often costs less than treating one serious injury. If regulators tighten requirements for toxic materials, companies and universities must act before rules change and not after the damage is done.
Every lab or manufacturer that depends on 2-Thienylacetyl Chloride knows that purity isn’t just a technical detail—it’s the backbone of good science and reliable results. This isn’t only for high-stakes pharmaceutical development or academic research; even mid-scale chemical processing can trip up without clarity about what exactly goes into a flask or reactor.
Whether in a university basement or a bustling industrial plant, folks who work with 2-Thienylacetyl Chloride soon find out that, yes, suppliers offer different grades. These run the gamut from “technical grade” for less sensitive reactions, to “analytical grade” and even “high purity” options when trace impurities can mess with downstream chemistry.
Technical grade—often cheaper—works for proof-of-concept reactions or small-batch synthesis where yield matters more than perfection. These batches might include a few percent of leftover starting reagents, maybe even some moisture. Try using this in complex syntheses or pharmaceutical processes, and the outcome becomes unpredictable. In my own work at a university lab, we once cut costs and grabbed a technical grade chemical, only to see our NMR peaks blurred and our project timeline stretch longer than anyone wanted.
On the other end, analytical and high purity batches lift that burden. Laboratories pay more, but they get peace of mind. These products promise tiny impurity levels—often under 0.5%, sometimes even lower. Reproducibility comes easier, especially when synthesizing building blocks or studying reaction mechanisms with sensitive instruments. These higher grades tend to come with documentation: you get safety data, certificates of analysis, and details about how the batch was stored and shipped.
In drug development, every atom counts. An impurity in 2-Thienylacetyl Chloride could slip through into a final active ingredient, tripping regulatory alarms and stalling approval. Environmental consequences add to the headache when impure chemicals release unexpected byproducts. The same story plays out in material science; a catalytic process could stall, or a polymerization might produce inferior properties all because the starting chemical wasn’t clean enough.
My peers in custom synthesis companies swap stories about inconsistent batches from unknown suppliers. They learned this lesson the hard way: test incoming materials, run a GC or HPLC, and keep a reference sample handy for trouble-shooting. They don’t gamble anymore, especially on large orders. Reliable suppliers become worth their weight in gold.
For those who have to take supply chain reliability into their own hands, start with an honest assessment: does this project tolerate impurities? If not, don’t settle. Request independent lab results and build long-term relationships with trustworthy suppliers. Push for transparency in batch testing. Keep good records across orders. Use smaller pilot batches to spot issues early—no need to waste a hundred liters.
Staying informed never gets old. Supplier websites might advertise purity specs, but nothing beats asking for the chromatogram or NMR data directly. In my experience, those who ask the tough questions up front build more dependable operations and avoid costly surprises later on.
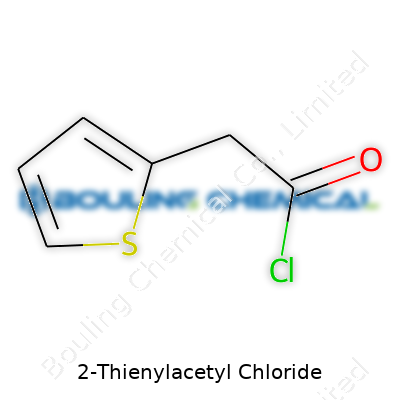
| Names | |
| Preferred IUPAC name | 2-(Thiophen-2-yl)acetyl chloride |
| Other names |
2-Thiopheneacetyl chloride Thiophene-2-acetyl chloride |
| Pronunciation | /tuː ˈθaɪ.nɪl əˈsiː.tɪl ˈklɔː.raɪd/ |
| Identifiers | |
| CAS Number | [39098-97-0] |
| Beilstein Reference | 1208956 |
| ChEBI | CHEBI:51522 |
| ChEMBL | CHEMBL19697 |
| ChemSpider | 122610 |
| DrugBank | DB07841 |
| ECHA InfoCard | 100.025.184 |
| EC Number | 211-545-2 |
| Gmelin Reference | 72642 |
| KEGG | C18927 |
| MeSH | D017740 |
| PubChem CID | 69849 |
| RTECS number | XN8575000 |
| UNII | WU3RLH859A |
| UN number | UN3431 |
| CompTox Dashboard (EPA) | DTXSID4094005 |
| Properties | |
| Chemical formula | C6H5ClOS |
| Molar mass | 176.64 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Pungent |
| Density | 1.32 g/mL |
| Solubility in water | Reacts violently |
| log P | 0.7 |
| Vapor pressure | 0.6 mmHg (20°C) |
| Acidity (pKa) | 14.9 |
| Basicity (pKb) | pKb: -7.4 |
| Magnetic susceptibility (χ) | -54.5e-6 cm³/mol |
| Refractive index (nD) | 1.5750 |
| Viscosity | 1.474 cP (25°C) |
| Dipole moment | 2.544 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 329.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -51.5 kJ/mol |
| Pharmacology | |
| ATC code | There is no ATC code assigned to '2-Thienylacetyl Chloride'. |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H314, H317, H411 |
| Precautionary statements | P261-P264-P271-P280-P301+P312-P305+P351+P338-P304+P340-P405-P501 |
| NFPA 704 (fire diamond) | 3-2-1-W |
| Flash point | 99°C |
| Autoignition temperature | Autoignition temperature: 590°C |
| Lethal dose or concentration | LD50 oral rat 300 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 1470 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 1 g |
| IDLH (Immediate danger) | N/D |
| Related compounds | |
| Related compounds |
2-Thienylacetic acid 2-Thienylacetamide 2-Thienylacetonitrile Thiophene Thiophene-2-carboxylic acid |