Back in the mid-20th century, folks in the chemical labs started looking for ways to add sulfur atoms into aromatic compounds. That’s where 2-thienylacetic acid found its groove. Research cropped up across Europe and North America, with chemists chasing new flavors of heterocyclic acetic acids for reaction studies and early drug leads. While the compound never got the crowd-pleasing buzz of bigger names like salicylic acid, a handful of researchers kept hammering out papers on 2-thienylacetic acid all through the 1960s and 1970s. Library shelves and digital archives still have those yellowed journal pages, a testament to dogged curiosity. Today’s organic labs pull it off the shelf for building blocks or as a benchmark for tweaking reaction methods, but those early pioneers laid the groundwork. Experience in research labs tells me you don’t need a blockbuster molecule to make useful chemistry.
2-Thienylacetic acid has a down-to-earth profile but a stubborn utility. With its thiophene core and a simple acetic tail, it offers a reliable starting point for making all sorts of sulfur-containing compounds. Chemists spot its use both in academic work and small-batch manufacturing. Bulk sales rarely match the volumes of common industrial acids, yet the right buyers keep it moving for reactions where a sulfur heterocycle makes all the difference. The chemical usually shows up as an off-white powder, sometimes a bit tan, depending on how carefully the manufacturer watches the process.
Pull that cap off a 2-thienylacetic acid tub and you’ll see fine, crystalline granules or a fluffy powder. Its molecular formula is C6H6O2S, with a molar mass around 142.18 g/mol. Once you get it out of the bottle, a faint, slightly sharp odor sometimes creeps up – not overwhelming, just a whisper of its sulfur atom. Laboratory thermometers clock its melting point between 74°C and 78°C, and the substance stays put at room temperature. Slide it into water and you’ll find modest solubility, a bit better in ethanol, dioxane, or ether. The acid character shows through with a pKa around 3.62, lining up with its mildly sour punch in solution.
Chemical supply houses stick labels on the bottles marking 2-thienylacetic acid with purity usually above 98%. You’ll spot the batch number, production date, molecular structure, and hazard codes – mainly skin and eye irritant warnings. For those keeping close records, common identifiers include CAS number 1918-77-0 and EC number 217-648-0. Labels also carry the UN transport number if the shipment crisscrosses borders or rides freight trucks. Laboratories pay heed to those details, as mislabeling sets everyone up for mistakes down the line. Most suppliers include a certificate of analysis to keep quality claims honest.
Synthesizing 2-thienylacetic acid involves a few reliable tricks. The most familiar approach walks through a Friedel-Crafts acylation, where thiophene reacts with chloroacetic acid under the firm hand of aluminum chloride or another Lewis acid. The reaction’s exotherm needs a careful touch since overheating brings side products and headaches. A cleaner alternative sometimes uses sodium thiophenolate and bromoacetic acid, letting nucleophilic substitution do the heavy lifting. Once the reaction wraps, there’s plenty of washing, filtration, and recrystallization before the pure acid comes out the other end. Scale-up means beefier glassware and stricter temperature control. Listening to old colleagues swap stories, it's clear that the winner in this game is always the technician paying close attention.
Once in hand, 2-thienylacetic acid opens the door to dozens of reactions. Common transformations include esterification with alcohols, amidation with amines, and coupling to form more elaborate heterocycles. The acetic acid side reacts in ways familiar from benzylic chemistry, but the thiophene moiety pitches in unique reactivity at the 2-position. For instance, halogenation there leads to halothiophenes with solid pharmaceutical value. Researchers sometimes oxidize it further to tweak biological activity or to anchor the compound into more complex architectures. In the hands of creative chemists, this little acid helps ring in all manner of chemical change.
In catalogues and research papers, names get swapped around. Spend a day poking through the literature and you’ll spot “2-thiophenylacetic acid,” “2-thienyl ethanoic acid,” or, written without spaces, “2thienylaceticacid.” A few suppliers simply call it “thiophene-2-acetic acid.” PubChem, ChemSpider, and other databases keep all those hats straight, which keeps researchers from accidentally ordering the wrong bottle. Though synonymous, these names point back to the same molecule and make searching a bit of a chore unless you know the landscape.
Working with 2-thienylacetic acid doesn’t require a hazmat suit, but it still needs respect. It can cause some eye and skin irritation, which means gloves and goggles stay on until everything’s cleaned up. Inhalation—or worse, ingesting it by accident—brings on mild nausea or irritation. Good ventilation rules the day, along with local exhaust hoods in busy organic labs. Disposal usually lands it in the aqueous organic waste stream, following well-worn environmental regulations. For storage, tight lids and cool, dry shelves keep the product solid and pure. Regulatory bodies like OSHA in the US and REACH in Europe have compliance frameworks, but 2-thienylacetic acid doesn’t trigger the toughest rules unless someone goes wild with amounts.
Pharmaceutical development leans on compounds like 2-thienylacetic acid to tinker with new drug backbones. Researchers exploring antiseizure drugs, anti-inflammatory candidates, and certain antibiotics have built on its framework. Agrochemical companies scout it for herbicide and fungicide leads, using the combination of sulfur and aromaticity to mess with plant biochemistry. Analytical labs sometimes use the acid as a reference for chromatographic calibrations or to build up training samples for GC-MS and HPLC runs. In material science, its derivatives play a role in polymers featuring thiophene units, with electrical or optoelectronic properties inching into solar or LED components.
For years, scientists kept poking at the thienylacetic scaffold for all sorts of research reasons. Some looked for new antibiotics, others pressed for cancer leads in the test tube. Folks in green chemistry try tweaking the preparation routes, shaving off waste and energy. R&D teams twisted the compound’s arms to build new ligands, surfactants, or ionic liquids. A few groups dove into the synthesis of photoactive compounds by linking the acid with electron-rich moieties. From my own bench days, seeing thienylacetic acid in the inventory always meant curiosity was about to meet glassware and pipettes.
The toxicity profile gets attention because adding sulfur often rings alarm bells. Data shows 2-thienylacetic acid doesn’t pack a dangerous wallop at the scale most labs use. Animal studies show only moderate acute toxicity, mostly hitting the digestive tract or causing mild irritation on contact. Toxicologists keep a healthy skepticism, so longer-term, high-dose studies run in parallel for any drug candidates build off the structure. In environmental testing, the acid breaks down reasonably fast, but spills into waterways need cleanup to avoid harm to aquatic life. My own reading finds few reports of chronic harm – just a need for good lab sense and solid personal protective gear.
Those who keep eyes on new technology spot the sulfur-rich thiophene ring showing up in modern applications, especially organic electronics. 2-Thienylacetic acid feeds into research around flexible solar cells, new photoconductors, and improved field-effect transistors. In pharmaceuticals, folks still see room for growth using the structure to chase central nervous system drugs or antimicrobial leads with fresh resistance profiles. Lab-scale synthesis keeps getting greener, with milder reagents and lower waste targets, which might tip large batch production from niche to mainstream. After decades spent as a workhorse for specialized projects, thienylacetic acid stands steady for anyone looking to build new molecules, tackle practical problems, or just keep learning about what sulfur can really do.
2-Thienylacetic acid may sound like a tongue-twister, but it holds a strong place in the chemistry world. This compound, built on a thiophene ring attached to an acetic acid side-chain, finds work in several corners of scientific research and manufacturing. What sets 2-thienylacetic acid apart is its sweet spot between reactivity and stability. It shows up most often as a building block for making more complex molecules.
Some call it small, but its effects in medicinal chemistry run wide. Pharmacists and researchers see 2-thienylacetic acid as a tool for assembling potential drugs. The molecule acts as a backbone for many types of pharmaceutical compounds, including anti-inflammatory drugs and agents used to treat neurological issues. Its structure lets chemists tack on different chemical groups, a flexibility that pushes drug discovery forward. I once shadowed a bench chemist screening a series of derivatives for painkillers—he started each experiment with grams of this exact acid.
Farming chemicals grow from just a handful of chemicals like this one. Agrochemical companies use 2-thienylacetic acid to create pesticides, herbicides, and plant growth regulators. These mixtures keep crops healthy and boost yields. Because of its relatively straightforward structure, it’s fast to modify. It offers what chemists crave: a reliable starting point.
2-Thienylacetic acid isn’t afraid to show up in unexpected places. In the lab where I interned during university, researchers tested thiophene-based monomers for new organic electronics. Small tweaks in the acid’s layout led to different electrical properties, including improved charge transfer in thin films. This kind of work has helped OLED screens and solar cells inch toward better efficiency. It’s a quiet helper in projects where every atom can nudge performance.
University labs love this molecule—walk down a hallway, you’ll probably find a bottle of it somewhere. Researchers depend on 2-thienylacetic acid to explore how different side chains affect molecular behavior. It shows up in synthetic routes for dyes, fluorescent probes, and specialty polymers. As students reach for it on the shelf, they gain a hands-on sense of how derivatives matter to final results.
Working with chemicals carries responsibility. Online lab registries and government health agencies (like the European Chemicals Agency) call for careful handling and safe disposal. 2-Thienylacetic acid may cause skin and eye irritation, so gloves and proper ventilation stay close at hand. Community standards encourage transparent documentation and adherence to safety protocols to cut risks and support repeatable results.
Although 2-thienylacetic acid supports a wide range of applications, the supply chain for specialty chemicals feels strain from global disruptions and high purity requirements. Academic teams and industry partners could invest in greener synthetic routes and broader training for technicians. Developing bio-based or renewable feedstocks represents another big step. With the right investment in safety and sustainability, this compound will keep supporting innovation in drug development, farming, and materials science.
2-Thienylacetic acid catches the eye in the world of organic chemistry, not just because of its unique ring structure but also for what it can teach us about heterocyclic compounds. On paper, the structure looks simple, but its significance goes deeper—especially for anyone diving into drug design, synthesis, or chemical education.
At its core, 2-Thienylacetic acid features a five-membered aromatic ring called thiophene. This ring holds four carbon atoms and one sulfur atom. Attached to the second carbon of this thiophene ring, you’ll find an acetic acid group, which marks the compound with both acidic and aromatic qualities. Written out, the formula stacks up as C6H6O2S. The basic backbone looks like this: a thiophene ring linked through its second position to a -CH2COOH group.
What makes 2-Thienylacetic acid stand out is its versatility in synthesis. I've seen this compound serve as a sturdy building block for more complex molecules in labs geared toward pharmaceuticals and fine chemicals. Thiophene’s stability, mixed with the reactivity of the acetic acid group, lets chemists branch into new compound families without fussing over too many side reactions.
For students, the structure of 2-Thienylacetic acid often pops up in lessons about aromatic chemistry and the role of sulfur atoms in molecular frameworks. It drives home the idea that swapping out a regular atom—like plugging in sulfur in the ring—can drastically shift a molecule’s behavior. Researchers lean on these insights to come up with medicines, dyes, and agricultural chemicals with fresh properties.
The conversation around any chemical also includes its safety and impact, not just its structure. In the handful of times I’ve worked with 2-Thienylacetic acid, I learned the hard way that even stable-looking compounds call for respect. Gloves, goggles, and good ventilation aren’t just boxes to tick—they keep accidents off the lab record.
This compound can cause a sharp smell, and some people react with skin or eye irritation if they handle it without protection. Waste disposal demands attention, too, since mishandling sulfur-based organics can have environmental consequences. Chemists now stay ahead of these risks by planning out safe workspaces and making sure every assistant knows the rules.
In my experience, the real push isn’t just learning to draw the chemical structure from memory. The bigger goal takes shape when schools and industries stress safer synthesis routes and greener waste management. Open discussions on alternatives—better solvents, improved purification steps, or bio-based starting materials—move the industry forward. By choosing smarter protocols at each step, labs can reduce both exposure and environmental footprint.
Using 2-Thienylacetic acid as a teaching tool or a feedstock doesn’t mean sticking with old habits. Newer generations of chemists question the status quo, picking up where previous protocols left off. Clean, accurate drawings of the molecule lay the groundwork, but it’s the attitude toward improvement that shapes real progress in chemistry.
Plenty of folks overlook the details that go into chemical storage—until something goes wrong. I’ve seen firsthand how routine lab work can go sideways when someone stashes a bottle of acid without reading up on its quirks. 2-Thienylacetic acid, for example, pops up in research labs thanks to its use in making pharmaceuticals and other chemicals. Simple mistakes while tucking it away can lead to ruined material or, worse, safety risks.
This compound isn’t flashy, but it still carries some responsibilities. At room temperature, 2-Thienylacetic acid takes a solid, powdery form. Leave that bottle open in a humid room, and you’ll invite moisture into the mix. Once, a chemist told me his sample clumped into a sticky mess just from careless sealing. Moisture exposure doesn’t just mess up the handling—it can start to change how pure the acid is, and that throws research off track before it even begins.
Nobody wants to babysit bottles all day, but 2-Thienylacetic acid calls for attention. Keep the container tight between uses. A screw-cap bottle with a sturdy seal works well, especially glass. Avoid stowing this acid in plastic that can react over time. I always advocate a simple dry cabinet or a proper chemical storage box. Don’t slide the bottle next to the sink or leave it in a damp lab corner—humidity sneaks in fast.
Temperature control saves more than people think. Most labs set storerooms around 20 to 25°C, which gives good peace of mind. Heat speeds up decomposition or unwanted chemical changes, and nobody wants the surprise of finding their samples altered. I once watched a newcomer place sensitive chemicals on a shelf right over a radiator, only to find them caked and discolored before the month was out. Lesson learned: treat chemicals with respect, and don't store them near heat sources, open flames, or in direct sunlight.
Clear labels save stress and help steer clear of accidents. Scrawled initials or half-torn tape won’t do—use real labels showing both chemical name and hazard information. That way, everyone gets the full picture in a hurry, even in a bustling workspace.
Never mix bottles of different acids, or worse, acids and bases, in the same bin. Storing chemicals together ups the risk of cross contamination or unexpected reactions. Years ago, an overstuffed bench led to a spill between two incompatible reagents, sending everyone out for fresh air. Baking safety into the storage setup means separating acids from bases and oxidizers, and reviewing the material safety data sheets (MSDS) for storage advice.
Unexpected spills and exposure happen, so good storage brings peace of mind. If something leaks or gets on skin, knowledge beats panic every time. Keep materials like gloves and eye protection within arm’s reach; don’t assume small quantities make for harmless handling. Emergency procedures, like eyewash stations and clean-up kits, hang around labs for a reason.
Solid habits keep mishaps rare. Rotate old stock out frequently, as old chemicals lose reliability. Check seals and containers often. Teach every new team member the whys, not just the hows. The value of chemical storage grows from real experience, passed along through small, careful steps. Nothing flashy—just a straightforward path to safer and more reliable science.
2-Thienylacetic acid doesn’t grab headlines, but it matters to chemists and researchers. Handling any fine powder or crystalline acid means staying aware of what can go wrong—the risks aren’t always obvious from a data sheet. Treating every batch like a possible threat helps because nobody wants unexpected skin rashes, coughing fits, or worse, a lab fire caused by careless handling.
This compound brings eye, skin, and respiratory hazards. Even if someone says, “It’s not as toxic as certain industrial acids,” that never means no protection. I’ve seen colleagues get lazy, skipping gloves just to weigh a sample or pour a little into a beaker. Almost every time, somebody ends up with irritated skin or eyes. You want a full set of protection—lab coat, splash goggles, and proper gloves (nitrile works well here). If the job might stir up dust or fumes, a fitted respirator keeps your lungs happy.
Dust stays on the bench long after the spill, and it spreads far quicker than you’d think. Ventilated hoods make a difference. In experience, labs where fume hoods saw regular use hardly ever complained about odd odors or coughs. If you’re pouring, dissolving, or heating 2-thienylacetic acid, always prefer the hood over an open-air bench. It protects everyone, not just the person right in front of the flask.
It’s tempting to grab the nearest rag after a minor spill, but nothing beats a plan that uses inert material like vermiculite. Sweep it up carefully and switch gloves right after. Changing out contaminated gloves keeps tiny amounts from landing on doorknobs or phones. Storage means a tightly sealed glass or HDPE bottle—straightforward, but a step people rush. I’ve seen containers not labeled or capped, attracting water vapor and turning the acid lumpy and hard to measure.
Material safety data sheets provide more than just regulatory comfort. Reading them is just the start; applying lessons is even more important. 2-Thienylacetic acid does not react violently with water or air, but storing away from oxidizers or strong bases makes sense from years of bench work. Accidents happen less often in labs where everyone respects the basics—and updates their storage methods as chemists cycle projects in and out.
Disposing of unused acid or contaminated gear can seem like a low-stakes problem. Pouring down the drain or throwing into the regular trash adds risks for the next person down the line—maybe janitorial staff or even wildlife. The smart play? Use a designated organic waste container. Our department saw a big drop in accidental chemical exposure after switching from generic trash bins to properly labeled hazardous material cans.
No rule works unless it’s followed. New lab members learn faster and work safer by watching experienced chemists go through every step—checking bottle labels, running down a mental checklist before starting. Seasoned scientists explain why a small shortcut often leads to larger trouble. Teamwork, questions, and admitting mistakes all play into better habits, both for handling this acid and for keeping the lab environment as safe as possible.
Prioritizing safety with every reagent shows care for accuracy, but more importantly, care for human lives. Years of laboratory work taught me that trust doesn’t just boost morale—it cuts down emergencies, property damage, and environmental impact. Every extra minute spent labeling, watching out for loose crystals, or alerting a colleague builds a culture far stronger than any warning sign or training manual alone.
2-Thienylacetic acid may not ring bells for most people, but in labs and chemical industries, this compound pops up all the time. Sometimes, the details make all the difference, especially in chemistry. Molecular weight sits right at the heart of dosing, synthesis, and even safety. For 2-Thienylacetic acid, the molecular weight clocks in at 156.18 g/mol. You get this total by adding up the atomic masses of each atom in the formula: C6H6O2S. Carbon, hydrogen, oxygen, and sulfur come together in a neat little package that moves more science than most people realize.
The number by itself looks dry, but experience in the lab has taught me that overlooking molecular weights turns good experiments into confusing messes. A precise measure means you scale reactions correctly. Add too much or too little reagent and you lose time, product, and in some cases, the whole experiment. When working with pharmaceuticals or crop compounds, those little missteps get expensive fast. Product engineers and researchers track weights down to the last decimal, knowing errors threaten not only results but budgets and reputations.
Handling thienyl derivatives lands you squarely in the real chemistry world: a full reaction flask, the hum of fume hoods, often with little room for mistakes. Safety relies on knowledge. A compound’s molecular weight underpins how much sits on your scale. Mess up that math, and toxic exposure or runaway reactions come next. Having the facts keeps people in one piece. I’ve seen production crews adjust their inventories based on these numbers, since raw material pricing and delivery count on the exact weight per batch.
Chemists are taught to use the periodic table like a roadmap. For 2-Thienylacetic acid, start with six carbons (6 x 12.01), six hydrogens (6 x 1.008), two oxygens (2 x 16.00), and a sulfur atom (32.07). Add those together: 72.06 (C) + 6.048 (H) + 32.00 (O) + 32.07 (S) lands on 156.18 g/mol, give or take small standard variations. In practice, scales and balances expect the number, computers want it, and paperwork doesn’t pass muster without it.
Having spent time in applied chemical analysis, I remember how many headaches a single decimal leftover could cause. Lags in product approval or recalls in pharmaceutical runs have, in real cases, traced back to simple molecular weight slip-ups. Beyond technical needs, accuracy builds trust. Consistent quality, effective doses, and compliant safety reports all start with a correct mass calculation. It’s not just about getting the work done but about standing behind the results with confidence.
Double-checking the math makes a difference. Most labs use reference databases or validated software for these calculations. Anyone handling chemicals, from new lab techs to veteran process managers, keeps the molecular weight at their fingertips. With responsibilities ranging from synthetic research to regulatory reporting, clear records and cross-checks keep things moving in the right direction. New chemists used to overlook these fine details until mistakes hit their own results or safety reviews. After a few close calls, people start to respect the calculation.
Beyond the world of textbooks, tools like ChemDraw, Sigma-Aldrich online catalogs, and curated industry databases speed along these checks. These resources update atomic weights and spot formula typos, which older reference books sometimes miss. Ongoing training pushes teams to respect precision. Peer-reviewed fact-checking, open discussion, and easy access to trusted sources all build a culture where accuracy grows. As industries evolve, the demand for reliable calculations grows right alongside, especially in pharmaceuticals, specialty chemicals, and environmental testing.
The focus on getting molecular weights right may seem basic, but every breakthrough, clean batch, and safety check starts with these simple numbers done well. For 2-Thienylacetic acid, 156.18 g/mol means more than a factoid — it’s a step toward better science and safer workplaces.
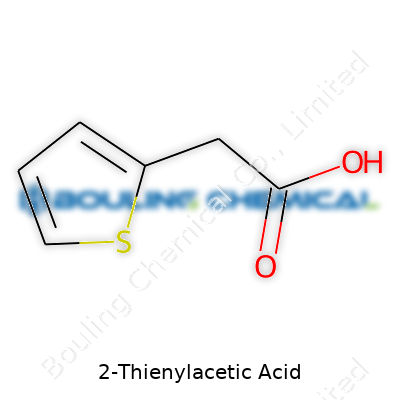
| Names | |
| Preferred IUPAC name | 2-(Thiophen-2-yl)acetic acid |
| Other names |
Thiophene-2-acetic acid T2AA Alpha-thiophen-2-ylacetic acid 2-Thiophenylacetic acid |
| Pronunciation | /tuː ˈθaɪ.ɪ.nɪl əˈsiː.tɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | [1918-77-0] |
| 3D model (JSmol) | `3D model (JSmol)` string for **2-Thienylacetic Acid**: ``` CC(=O)C1=CC=CS1 ``` |
| Beilstein Reference | 1202035 |
| ChEBI | CHEBI:19301 |
| ChEMBL | CHEMBL418356 |
| ChemSpider | 77910 |
| DrugBank | DB08325 |
| ECHA InfoCard | 13b82661-df0b-484c-86b3-b2c70f6a0010 |
| EC Number | 2.3.1.6 |
| Gmelin Reference | Gmelin 83690 |
| KEGG | C06222 |
| MeSH | D013954 |
| PubChem CID | 6955 |
| RTECS number | AJ2625000 |
| UNII | 1S8T8E5C7U |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | DTXSID5037668 |
| Properties | |
| Chemical formula | C6H6O2S |
| Molar mass | 140.17 g/mol |
| Appearance | White to light brown crystalline powder |
| Odor | Odorless |
| Density | 1.33 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.09 |
| Vapor pressure | 0.0000165 mmHg (25°C) |
| Acidity (pKa) | 3.5 |
| Basicity (pKb) | 2.89 |
| Magnetic susceptibility (χ) | -51.6·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.573 |
| Dipole moment | 1.97 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 216.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −120.6 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -694.8 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P270, P280, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 121°C |
| Autoignition temperature | 410°C |
| Lethal dose or concentration | LD50 oral rat 2800 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 500 mg/kg |
| NIOSH | Not listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | 250g |
| Related compounds | |
| Related compounds |
Thiophene Thiophene-2-carboxylic acid 2-Bromoacetic acid Phenylacetic acid Furan-2-acetic acid |