Scientists first brought 2-Thenoyltrifluoroacetone (TTFA) into laboratories during the mid-20th century, chasing after stronger, more flexible chelating agents. The world of coordination chemistry craved molecules that could pull toxic metals out of solutions or tease apart unravel-ed complexes. TTFA grew from the larger diketone family. Researchers in both Eastern and Western labs recognized it—first for its ability to bind metals like iron and copper, then later as a tool for studying mitochondrial biochemistry. Throughout the 1960s and 1970s, TTFA’s usefulness in understanding electron transport began turning up in journals. Once word spread about its role as a mitochondrial respiration inhibitor, the compound found new life in medical and biochemical research circles.
At its core, 2-Thenoyltrifluoroacetone combines a thenoyl group with a trifluoroacetyl group—giving it a structure unlike most common lab reagents. This chemical serves as a specialty product, sitting on benches in academic, industrial, and pharmaceutical settings. One finds it used especially for analytical chemistry, as a ligand in separation processes, and as a research tool probing processes ranging from catalysis to cellular respiration. Companies package it in glass bottles or specialty pouches, ensuring only the calculated dose hits the reaction whenever it ships to end users.
Presented as a yellow, crystalline powder, 2-Thenoyltrifluoroacetone offers up a slight characteristic odor. It resists dissolving in water, instead favoring organic solvents like ethanol, chloroform, and ether—a reflection of its non-polar structure. Melting sits around 52–56°C, a manageable range for most lab work. Chemically, TTFA stands out as a β-diketone showing strong electron-withdrawing effects thanks to the trifluoromethyl group. This shift in electron density pushes the molecule to act as a formidable chelator, especially with transition metals. Any flask holding TTFA in solution should stay away from bright daylight, since prolonged light exposure slowly breaks down the active diketone structure.
Manufacturers typically specify 2-Thenoyltrifluoroacetone at a purity of 97–99%. Labels clearly note CAS Number 326-91-0, molecular formula C8H5F3O2S, and molecular weight of 222.19 g/mol. Tech sheets include melting point, solubility data in various solvents, and guidance on stable storage (dry and dark, ideally below room temperature). Packaging always includes clear hazard statements and pictograms under GHS standards, noting the need for goggles and gloves in direct handling.
Producing TTFA isn’t a quick one-pot trick but involves sequential acylation and condensation reactions. Chemists treat 2-acetylthiophene with trifluoroacetic anhydride in the presence of a base, driving enol formation and then pulling off excess reagents through careful purification, often column chromatography or recrystallization. The trick lies in balancing reaction times and temperatures, since both the thenoyl group and the active methylene want to react with more than just the designated partners unless reaction conditions stay tight. Reproducibility depends on careful control, particularly complete exclusion of moisture and oxygen during critical stages.
This molecule shows up as a workhorse in the synthesis of coordination complexes. The enolizable hydrogens, flanked by carbonyls, let it snap onto metal ions (like Fe3+, Cu2+, or Ni2+), yielding colorful chelates that help both in analytical detection and in catalysis. The trifluoromethyl group adds bulk and electron-withdrawing clout, so when TTFA reacts, products come out more stable and less likely to decompose in air than some related β-diketones deliver. TTFA can also undergo typical oxidative transformations or halogenation at reactive sites, giving rise to analogs for exploring structure-activity relationships in medicinal chemistry research.
Finding 2-Thenoyltrifluoroacetone on supplier websites means searching its many aliases—TTFA, 1,1,1-Trifluoro-4-(2-thienyl)-2,4-butanedione, or even “trifluoroacetylthienylacetone” in some catalogs. Literature also refers to it under its systematic nomenclature, sometimes varying how they spell the thiophene attachment site. Chemical Abstracts Service holds it as number 326-91-0 regardless of brand or global market. Some suppliers market it as simply “β-diketone TTFA” for easier reference, especially among those shopping for analytical standards or fine chemicals.
Working with TTFA means keeping safety a daily habit. Users wear gloves, splash-proof goggles, and lab coats, as TTFA’s dust can irritate skin and eyes, especially after longer exposures. Fume hoods stay in routine operation, since inhaling even small amounts of vaporized powder or solvent-laden fumes brings on nose and throat irritation. The trifluoromethyl group doesn’t make for an acute hazard, but chronic exposure studies in animals push users toward smart handling: closed systems, meticulous cleanup, and chemical waste segregated per local rules keep accidental environmental releases in check. Any spilled product gets swept with damp towels, never a dry broom, because even powders linger long after a surface wipe-down.
Industries and research labs put TTFA to work in plenty of fields. Analytical chemists use its chelating power to separate and quantify trace metals in complex samples, sometimes by liquid-liquid extraction or by forming colorimetric complexes for spectrophotometry. Cell biologists value TTFA as a mitochondrial complex II inhibitor, slowing electron transport and revealing new wrinkles in metabolism, cellular respiration, and drug response. Others add TTFA to catalysts, chasing stronger selectivity or tuning reactivity thanks to the stabilizing effects of trifluoromethylation. Academic labs testing new organic synthesis methods keep TTFA around as a model substrate, since it responds cleanly under many reaction conditions (oxidations, reductions, cross-couplings) and hints at what fluorine does to reaction outcomes.
Over decades, TTFA’s presence in published literature says a lot about its staying power. In the 1970s, TTFA helped scientists explain how mitochondria fail when faced with poison or disease—a jumpstart for the field of mitochondrial medicine. More recent years see TTFA as a staple for screening new metal complexes, especially for sensors or environmental detectors. Pharma companies dip into TTFA’s structure to design drugs that modulate metal ions in the body, a strategy catching interest for both rare genetic diseases and common age-related ones. Research groups explore TTFA’s performance in solid phase extraction, environmental cleanup, and even next-generation battery materials where chelation or fluoride tolerance matters.
Short-term TTFA exposures usually bring mild irritation, but deeper research on laboratory animals tells a more complicated story. Chronic dosing at high levels disrupts mitochondrial function—showing up as liver and kidney effects, along with changes in energy metabolism. Cellular assays see a dip in ATP levels as cells struggle with impaired electron flow. TTFA’s impact on aquatic life draws scrutiny in environmental studies, especially when labs flush spent TTFA solutions down drains. Regulatory agencies cite animal data when setting permissible exposure limits, pushing for careful ventilation and personal protective equipment during use. Overall, while acute toxicity in humans stays relatively low, the mitochondria-targeting mode of action calls for extra respect.
Signs point to TTFA sticking around, especially with growing interest in both chemical separations and medical research targeting metabolism. Industry trends see pharmaceutical companies looking for new mitochondrial modulators, inspired by aging research and rare disease treatment. Meanwhile, analytical chemists working in environmental or food safety analytics find that TTFA and its analogs help detect ever-lower concentrations of toxic metals—meeting the world’s rising regulatory standards. Chemists aiming for greener processes also experiment with TTFA in recyclable or reusable catalysts. As new generations chase breakthroughs in bioenergetics, sustainable extraction, or catalysis, TTFA looks ready to keep its place as a quietly essential molecule, as long as the right safety standards, disposal practices, and fresh ideas keep evolving along with it.
Step into any college chemistry lab or a pharmaceutical company’s research wing and 2-Thenoyltrifluoroacetone, or TTA for short, might not sit on the front bench, but it's certainly doing some heavy lifting behind the scenes. I remember the first time I crossed paths with TTA—a lab supervisor dropped the name during an experiment involving rare earth elements. It sounded bulky and mysterious. Digging deeper, I realized this compound quietly solves a range of scientific challenges.
TTA works as a chelating agent. This means it forms stable complexes with metal ions by binding them tightly. You’ll find this ability handy in separating valuable metals like lanthanides and actinides, especially when clean separation really matters. Think about the demand in high-performance electronics or cutting-edge medical diagnostics—those fields often depend on extremely pure materials. Chemists use TTA to grab hold of one kind of metal ion while letting the others slip through, almost like letting one friend into a party while the others stay at the door.
The importance ramps up in nuclear technology. Processing nuclear fuel throws up a tough separation problem, and TTA serves as a trusted tool for isolating uranium, plutonium, and other elements. Mistakes here aren’t just costly; they threaten safety, so a reliable method matters. That makes TTA more than a lab curiosity—it becomes part of keeping real people safe and productive.
Older textbooks put TTA in the pages about metal analysis, but the story doesn’t stop there. In recent years, scientists started using it in new places. TTA shines in photoluminescent materials—the glowing powders and coatings that make signs visible in the dark or give medical imaging contrast agents their vibrant kick. These applications rest on the way TTA coordinates with rare earth ions, helping unleash unique optical effects. You won’t notice TTA working, but if you’ve marveled at glowing detector screens or learned about novel cancer treatment tracers, you’ve glimpsed its influence.
TTA also helps in the oil and gas sector. Geologists rely on it for selective extraction during exploration projects, where identifying strategic metals can drive an entire mining operation. Instead of going through tons of rock, chemists mix TTA into a solution and watch as it isolates only the metal in question. Time and money saved, waste cut down—that’s a win in itself.
Not everything about TTA smells like roses. Careless handling of fluorinated compounds, including TTA, can mean trouble for the environment and for people exposed over the long run. Every chemist hears the lecture about proper containment and disposal, but labs sometimes cut corners under budget pressure or during hectic periods. Regulatory agencies set rules, but enforcement doesn’t always keep up. Companies and universities ought to invest in safer alternatives or improve reclamation and recycling of materials where practical. Green chemistry principles—reducing waste and looking for less toxic substitutes—deserve a front-row spot in research agendas, both for safety and future innovation.
TTA catches attention because it's practical, solves gritty problems, and fits the pace of modern science. Used responsibly, it keeps the wheels turning for tech, medicine, and industry. With another look at safety practices and a steady dose of curiosity, TTA and its cousins might keep opening new doors in the lab—and outside it—for years to come.
Storing chemicals at home or in the lab brings real headaches, if you’ve ever handled stuff like 2-Thenoyltrifluoroacetone. Bottles labeled with long, hard-to-pronounce names might just look like extra work, but the headache grows when these bottles end up in the wrong spot. You can’t treat them like baking soda or stock them next to the window. Strong chemicals, especially ones used in analytical labs like 2-Thenoyltrifluoroacetone, can act up if you ignore their quirks.
Exposure to light, heat, and air can throw off the stability of 2-Thenoyltrifluoroacetone. The trifluoro group and the ketone functional group don’t mix well with uncontrolled moisture or high temperatures. Bottles that look fine on Monday might turn dangerous by Friday if they’re picked up after sitting too close to a heat source. Extra caution keeps your experiment results in check and prevents waste, too. Ruined batches mean buying more supplies, filling up waste bins, and maybe even tossing a whole week’s work down the drain.
Physical chemistry teachers often forget to tell new lab members that keeping these compounds at room temperature isn’t just about “reducing volatility.” The way this stuff reacts comes from real-world chemistry, where elevated temperature speeds up decomposition and crumbly, unstable residues. That’s happened more than once in crowded shared labs I’ve worked in, and the smell tells you all you need to know. Refrigeration below 20°C keeps the bottles quiet—most lab folks aim for 2–8°C in a typical chemical fridge, set aside from food and drinks.
Light doesn’t just fade your favorite t-shirt. Light-sensitive compounds, especially those with complex rings and groups like 2-Thenoyltrifluoroacetone, slowly break down or discolor under ordinary bulbs. Forgetting this means walking in on a yellow or brown mess instead of the pale, slightly oily liquid you expected. Containers need a solid seal and a dark or amber bottle, plain and simple.
Air and moisture creep in fast, especially if bottles aren’t closed right. The air’s not just harmless oxygen; it comes mixed with water vapor, and those small sneaky amounts set off hydrolysis or other unwanted side reactions. You notice a chemical’s gone off when the experiment result goes sideways, and nobody wants to troubleshoot mysterious peaks or weird colors for hours. Using proper caps and storing everything upright slows down the attack.
Every busy bench gets messy by Friday, but keeping fresh silica gel packs near the shelves, labeling open dates, and not letting bottles hop from hot to cold spots pays off over time. Nobody enjoys coming back to chunky, brown goop where a smooth liquid should be. Safety goggles and gloves aren’t enough if careless storage turns a simple weigh-and-mix into a chemical incident.
Practicing good habits—rotating stock, recording opening dates, checking for warning signs—doesn’t signal inexperience. Instead, it saves headaches, protects results, and skips emergency runs for new stock. If you have a walk-in chemical fridge, use it. Don’t toss bottles on top of old ones or stack them where a knocked bottle ruins the next batch for everyone. These basic steps aren’t about rules. They’re reminders from anyone who’s lost a good experiment to careless storage—or seen a bottle’s insides bubble when it shouldn’t.
2-Thenoyltrifluoroacetone, usually abbreviated as TTA, pops up in science as a chelating agent. In plainer words, TTA grabs onto metal ions and makes them easier to extract, especially from mixtures where these ions hide deep. It's not a household name, but it’s found in research labs, chemical plants, and sometimes in the pursuit of rare earth elements or nuclear fuel work.
The word “toxic” gets thrown around, sometimes a bit too loosely. For many chemicals, toxicity depends on the dose, how people interact with the substance, and for how long. TTA isn’t what you’d call harmless. A quick look at its safety data shows health warnings that make you pause: skin irritation, possible eye damage, potential for respiratory distress if inhaled, and trouble in rivers or ecosystems if it leaks out. The body doesn’t shrug it off.
Few people outside science circles bump into TTA, but being rare in daily life doesn’t wipe out the danger. If you work in a lab, TTA often comes as a yellow powder or crystals, easy to spill, and the dust floats far. Synthetic gloves, goggles, and fume hoods aren’t suggestions—they’re shields. My own lab days hammered this point home: someone gets careless, brushes a sleeve through a powder, then rubs an itch near the eyes—redness or burning usually follows.
Studies place TTA in a category similar to many other tri-fluorinated organics—it can irritate the skin or cause trouble if inhaled. Some animal studies detail changes to the liver and kidneys after exposure, but those came at higher doses than most humans would ever meet unless something went wrong in a lab.
Ignoring lab chemicals just because most people never see them misses the point. Chemical plants vent fumes and run the risk of spills; environmental agencies keep tabs for a reason. Sometimes waste skips treatment, and rivers or soil suffer. The molecules in TTA resist breaking down, sticking around in ground or water longer than folks might expect. Local wildlife doesn’t stand a chance if enough of the stuff comes their way.
We’ve all seen stories of fish kills, strange foam on lakes, or farmland that won’t grow. Even if TTA hasn’t grabbed headlines, its family of chemicals often does. The lesson sticks: keeping “lab-only” chemicals away from air, water, and food doesn’t just help scientists–it protects everyone.
Responsible handling sits at the center. Labs that use TTA lock up stocks, set clear rules about waste, and trace every gram used so nothing escapes by accident. It helps when regulators demand more than paperwork—actually visiting labs, checking waste disposal, holding companies to account.
On a larger scale, research shouldn’t just focus on what TTA can do for industry, but what safer alternatives might tackle the same jobs. Green chemistry isn’t just a buzzword—it nudges everyone to find cleaner, softer-edged tools. For now, simple steps like airtight storage, strong fume extraction, and end-to-end waste tracking really do cut the risks.
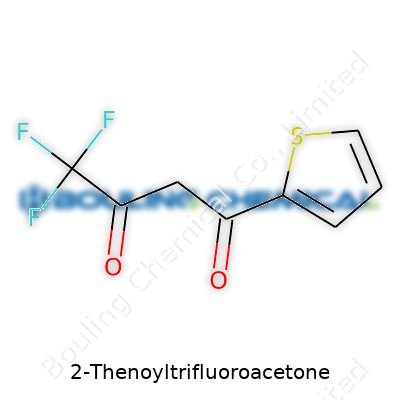
2-Thenoyltrifluoroacetone ranks among those chemicals you bump into if you’ve spent time in research labs or prowled the shelves in a university storeroom. Its name is a mouthful, but break it down, and you’re looking at a β-diketone with a thienyl ring on one side and a trifluoromethyl group on the other. For people familiar with organic synthesis, this combo has all the makings for intense coloration when reacting with metal ions, which explains why it gets picked up for spectrophotometry work and metal extraction. Let's get down to its specifics.
Every time I pulled 2-Thenoyltrifluoroacetone off the shelf, the label glared back with two critical figures: C8H5F3O2S for its chemical formula, and 222.19 g/mol stamped as its molecular weight. The formula packs eight carbons, five hydrogens, three fluorines, two oxygens, and a sulfur atom—a snapshot of its structure. Those three fluorine atoms, lined up on a single carbon, don’t just sit there for show; they crank up the electron withdraw, which shifts the acidity of the central hydrogen, and that’s precisely what makes this molecule jump into action during complex formation with metals.
As a past grad student poking around with extraction solvents, I remember how easy it was to tell which bottle contained thfa (as the shorthand goes) by just looking at that trifluoromethyl group printed on every label. No matter what the bottle said, it was always about those atoms—the tiny blueprint used to explain everything from its reactivity to how readily it dissolves in various organic solvents like chloroform.
Plenty of chemists might gloss over those two numbers, but in the trenches, mistakes can cost you your whole week’s work. Calculating how much to weigh or mix relies on that 222.19 g/mol figure. Preparing a stoichiometric solution for complexometric titrations or even metal detection means grabbing a calculator and the precise molar mass, or else accuracy goes out the window.
Once, during a rush to get through an experiment before a long weekend, I miscalculated the molecular weight because I jumbled up numbers from another ligand. Result? Misidentified the quantity and wound up with a dud for spectrophotometry—and didn’t get the sharp peaks needed. It taught me once and for all that no detail is too small. The formula tells you what you’re dealing with, but the molecular weight tells you how much you need in the real world.
Keeping lab work on track takes more than careful reading; using standardized digital resources is key. In my own experience, cross-checking with modern digital chemical databases like PubChem has become non-negotiable. A quick search for “2-Thenoyltrifluoroacetone” delivers not just the formula and weight, but also melting points, safety hazards, and solubility data to avoid slip-ups. Labs with strict protocols run far smoother, and so does research output. A little preparation before each experiment, double-checking numbers, and updating the bottle labels prevent setbacks and boost confidence—something every student or professional can appreciate.
Chemical formulas and weights aren’t just numbers stuck on bottles. They’re the start point for every serious scientific endeavor, and knowing how to use them makes the difference between an educated guess and a scientific outcome you can trust.
2-Thenoyltrifluoroacetone, better known in the lab as TTA, turns up in more research projects and industry processes than most people realize. Folks use it for metal ion extraction, NMR spectroscopy, and making laser dyes. This chemical looks unassuming, but it packs enough punch to make safety a serious conversation. TTA can irritate the skin, eyes, and even lungs, especially if it goes airborne or gets on unprotected hands. I once saw a careless mix-up with TTA end with someone eager for the eyewash station — and that memory sticks.
Labels warn about hazards, but they can’t enforce habits. Good sense matters more than pretty posters. Wearing gloves, goggles, and a lab coat isn’t optional. Some think skipping gloves saves time during a rushed experiment; lessons tend to arrive fast and stinging. TTA can absorb into the skin. Once, I had to spend an awkward half day in urgent care after a spill during a grad school project — stubbornness wasn’t worth it. Fume hoods belong in the conversation too. Spilling or even just weighing out TTA on an open bench stirs up dust that no one wants in their lungs.
TTA doesn’t break down quietly if left around. Keep it sealed and away from light and moisture. At our lab, every employee knew that a leaky bottle or even a loose cap risked contamination for the whole building. It’s common sense to stash this chemical in a flammable storage cabinet, segregated from acids and bases. Some folks still keep hazardous bottles close to their workspace out of convenience, but shortcuts belong in recipe books, not in chemical storage.
Flushing TTA down the drain? Bad idea. This stuff harms aquatic life and sticks around in the environment. Once, a research partner tried a shortcut to save time, and the waste treatment folks weren’t pleased — nobody wants hazardous waste fines. Every lab’s hazardous waste protocol looks different, but the basics stay the same. Pour the leftover TTA, along with any contaminated gloves, pipette tips, or glassware, into a proper hazardous waste container. Label the waste clearly, and don’t let it sit for weeks in the corner. These containers need pickup by professionals who understand the chemical’s quirks; they’ll incinerate or treat it through chemical decomposition. Municipal landfills, dumpsters, and the regular trash shouldn’t see even a hint of TTA.
Open discussions in labs about chemical protocols save more than time; they prevent emergencies. I always recommend regular safety meetings, not just the annual ones, because frequent reminders set a stronger culture. Training new staff, especially undergraduates fresh to the bench, guards against sloppy practices that might linger for years. Institutions could do more — funding better ventilation or providing easier access to up-to-date safety data — but in the end, personal attention to detail stands as the last line of defense.
TTA’s risks don’t fade after the experiment ends. People managing labs and facilities owe their team and community more than adhering to regulations: they set the tone for responsible science. Mistakes echo outward. Good handling and disposal habits start with one person, but ripple out, raising everyone’s standards. The best labs I’ve seen treat every small bottle of TTA with all the respect of a much larger threat — and in that approach, they keep their people, research, and environment safe.

| Names | |
| Preferred IUPAC name | 1,1,1-Trifluoro-4-(2-thienyl)butane-2,4-dione |
| Other names |
TTFA 2-TFA 2-Trifluoroacetylthienyl 2-Thienyltrifluoroacetone |
| Pronunciation | /tuː-θɪˈnɔɪl.trɪˌflʊə.roʊ.əˈsiː.tən/ |
| Identifiers | |
| CAS Number | 326-91-0 |
| Beilstein Reference | 1208732 |
| ChEBI | CHEBI:39068 |
| ChEMBL | CHEMBL145070 |
| ChemSpider | 11453 |
| DrugBank | DB01973 |
| ECHA InfoCard | 100.016.425 |
| EC Number | 203-977-6 |
| Gmelin Reference | 75040 |
| KEGG | C06424 |
| MeSH | D013808 |
| PubChem CID | 66447 |
| RTECS number | XZ3150000 |
| UNII | ns6t8c1596 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DJ1UHQ875D |
| Properties | |
| Chemical formula | C8H5F3O2S |
| Molar mass | 222.17 g/mol |
| Appearance | White to pale yellow crystalline powder |
| Odor | Odorless |
| Density | 1.454 g/mL at 25 °C (lit.) |
| Solubility in water | slightly soluble |
| log P | 1.8 |
| Vapor pressure | 0.00029 mmHg (25°C) |
| Acidity (pKa) | 6.78 |
| Basicity (pKb) | 6.18 |
| Magnetic susceptibility (χ) | -59.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.497 |
| Viscosity | 0.7800 cP (20°C) |
| Dipole moment | 4.29 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 372.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −1125.6 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3725 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H319 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 104°C |
| Autoignition temperature | 617°C |
| Lethal dose or concentration | LD50 (oral, rat): 5370 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 720 mg/kg |
| NIOSH | SN8850000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 mg/m3 |
| Related compounds | |
| Related compounds |
Acetylacetone Benzoyltrifluoroacetone Dipivaloylmethane Hexafluoroacetylacetone Trifluoroacetylacetone |