Folks working in organic chemistry recognize 2-thenoic acid as a small but interesting piece of the larger heterocyclic puzzle. Chemists first isolated this compound over a century ago, pushing through the evolving world of aromatic chemistry. At the time, curiosity about sulfur-containing rings such as thiophene opened up new directions for pharmaceutical and materials research. Early methods usually involved harsh oxidation of thiophene derivatives. As synthetic strategies matured, scientists found more efficient routes, letting the compound become a basic building block for many experiments. Labs around the world relied on improved preparation and purification, pushing 2-thenoic acid into use across academic and industrial fields.
Anyone who works with 2-thenoic acid sees first-hand that it usually arrives as a pale, needle-like solid or a faintly yellow crystalline powder. At the bench, it offers a mix of convenience and challenge: stability for storage, but moderate sensitivity to air and light. Chemists favor it in pilot synthetics and as a reference standard in spectral studies. As with many heteroaromatic acids, the tangible impact lies in its promise for fine chemicals, drug design, and sometimes even agrochemical research. Reputed suppliers often guarantee a minimum purity above 98% for sensitive uses, lowering the risk of side-reactions from hidden contaminants.
2-thenoic acid, formally known as thiophene-2-carboxylic acid, weighs in at 128.14 g/mol, presenting itself with a melting range generally tight around 138–140°C. Its faint smell sometimes hints at burnt sulfur. The molecule comes with a planar, rigid ring—a feature that affects how it interacts in reactions. Its acidity (pKa near 3.0) gives it a sharper proton release compared to benzoic acid, often making it more reactive in certain esterification and salt-formation conditions. Water barely dissolves it, but common polar organics like ethanol or acetone handle the task. The sulfur atom on the ring impacts electron flow, so the molecule’s chemical behavior sometimes surprises less experienced chemists. People working in analytical techniques treat its structure as a good test for developing NMR and IR spectral assignments in sulfur-heterocycles.
Manufacturers tend to list 2-thenoic acid under a variety of labels: CAS number 98-59-9, high-performance-grade, or sometimes as “analytical quality.” Specifications from reputable vendors spell out features like content not less than 98%, detailed trace levels of water and heavy metals, and clear batch lot records. Safety data show up on every certificate of analysis—flash point, storage recommendations, and critical signal words required by GHS labeling standards. Anyone purchasing for research pays close attention to the appearance and exact mass data to confirm authenticity. The best suppliers back up listed properties with lot-wise HPLC or GC trace profiles.
Lab teams often reach for oxidation of thiophene as a go-to route, using potassium permanganate or similar oxidizers. Some research pushed for eco-friendly alternatives using aqueous hydrogen peroxide and catalytic conditions. Depending on the route, yields can reach above 70%, but the isolation sometimes demands precise temperature and pH control. Milder modifications using carboxylation of lithiated thiophene lower the formation of side-products. In every route, water workups, filtration, and careful purification by recrystallization mean reliable product in hand, ready for further transformations. My own time in the lab showed that even with clean reagents, sharp attention during oxidation keeps tar byproducts under control—a lesson anyone preparing the acid quickly learns.
This compound’s carboxylic handle makes it a starting point for a lot of downstream chemistry. It reacts cleanly with alcohols and amines for ester or amide formation under well-controlled conditions. The thiophene ring’s electron distribution makes electrophilic substitutions a bit more directed than in non-sulfur rings, so halogenation or nitration usually targets the 5-position, giving access to versatile intermediates. Reduction of the acid leads to the corresponding alcohol, while decarboxylation steps strip out functional groups, setting up further skeletal changes. I’ve watched high-yielding cross-coupling reactions, where copper or palladium catalysts help to stitch the ring into larger structures, especially useful for building out active pharmaceutical ingredients or engineering new material surfaces. Each pathway offers specific benefits for tuning solubility, biological activity, or optical properties in research or final applications.
Anyone sifting through literature or ordering stock comes across a mix of synonyms: thiophene-2-carboxylic acid, 2-carboxythiophene, or occasionally just “2-thiophene acid.” Some catalogs slot it under systematic IUPAC labels, but street names in the lab often shorten terminology to make communication easier. Across regulatory filings, the CAS registry number (98-59-9) helps avoid mix-ups. People handling inventory records or tracking down research references need to keep an eye on these subtle shifts in naming, since cross-referencing old publications or regulatory approvals can bring surprises.
Responsibility in the lab starts with understanding risk, and 2-thenoic acid comes with its own set of precautions. Inhalation or contact with dust sometimes causes mild irritation, though not typically severe reactions unless exposure runs long. Safety data sheets spell out PPE guidelines: gloves, goggles, and working under a hood during handling and weighing, especially for larger-scale runs. Waste and residue disposal counts for a lot—local regulations call for use of secondary containment and collection as organic acid waste. From an operational side, storage in tightly closed, light-protected containers at room temperature keeps decomposition at bay. Direct experience showed that following these simple but important steps, especially in busy teaching labs, cuts down accidents and lets researchers focus on results.
Practical value for 2-thenoic acid shows up most in specialty chemicals and as an intermediate for drug molecules. Medicinal chemists build on the thiophene core to explore anti-inflammatory and antimicrobial potential, often by attaching further groups to the ring or acid function. Some crop protection products use this backbone, exploiting its compatibility with bioactive substituents. Dye synthesis and polymer chemistry use the compound as a starting point for light-sensitive or conducting materials—key steps in developing specialty sensors, organic semiconductors, and some experimental solar cell materials. My own work ran into it as a linker in library synthesis, giving lots of chances to study how the heterocycle’s lone electron pairs interact with metals or hydrogen-boners, a lesson in how little changes at the molecular level ripple through to big shifts in solubility or cell activity.
Anyone following patent literature or new articles sees that researchers keep pushing 2-thenoic acid into fresh territory. The ongoing push for green chemistry drives inventors to seek cleaner oxidants or direct electrochemical synthesis. Industry partnerships often sponsor studies to upgrade existing routes, cut out hazardous intermediates, or boost yield and selectivity, ultimately aiming for more cost-effective mass production. Biomedical teams, drawn by the sulfur ring’s impact on bioavailability, use 2-thenoic acid and its derivatives to chase leads against challenging bacterial or cancer models. Polymer scientists look for new coupling routes to create advanced materials. Each new modification tricks out fresh reactivity or opens more application fields. Enough time in the trenches shows that even marginal upgrades in yield or selectivity can reshape a research program or move a process closer to industrial scale.
Animal and cell model data point to low acute toxicity for 2-thenoic acid, but caution dictates keeping exposures limited in both industrial and academic settings. Studies track eye and skin irritation, with results aligning it with mild-to-moderate hazard in concentrated form. Chronic exposure data mainly draw from workplace settings, and strong monitoring programs help keep risks controlled. Regulatory agencies consider both environmental and bioaccumulative potential, usually finding disposal in moderate hazard range alongside other carboxylic acids. The scientific community keeps an eye on any new findings about metabolite formation or long-term health impacts. In settings aiming for zero accident records, folks invest in proper fumehoods, double-layer gloves, and air monitoring—common sense tactics but ones that matter for peace of mind and good lab morale.
Looking out ahead, 2-thenoic acid stands poised to play a stronger role in specialty synthesis, advanced materials, and possibly as a feedstock in emerging drug classes. Industry needs for lower-toxicity building blocks—especially those offering packing density and functional group tolerance—drive research to adapt core structures and invent more sustainable routes. Biotechnologists explore engineered metabolic pathways to generate sulfur-heterocycles with less waste and energy. As drug discovery expands to cover new disease targets, the flexibility of the thiophene ring sits well with property-optimization workflows, letting teams adjust solubility or pharmacokinetics with targeted substitutions. Growth in organic electronics hints at new space for the acid as a precursor for rare ligands, thin films, or designer nano-assemblies. From my own perspective, reality checks come from the bench: well-labelled bottles, clear protocols, thoughtful handling, and a willingness to share hard-learned tricks keep this age-old molecule current, letting the next generation push the boundaries even further.
2-Thenoic acid may sound like something only a chemist cares about, but this small molecule supports bigger things in research and industry. With its sulfur-rich five-membered ring, it belongs to the family of heterocyclic compounds that have influenced pharmaceutical discoveries and advanced chemical reactions.
Many people who pick up their prescriptions probably never think about the mountain of work behind the scenes. 2-Thenoic acid steps in during drug discovery as a starting point and building block. Chemists attach, alter, and test this core to shape entirely different medicines for the treatment of infection, epilepsy, and other conditions. Its unique shape offers chemical diversity, and in the world of drug design, variety matters. Derivatives from 2-thenoic acid have shown activity against bacteria, fungi, and even cancer cells. One journal article in the European Journal of Medicinal Chemistry explored how tweaking this thiazole ring structure helped develop stronger antibacterial molecules, pointing the way toward more effective drugs as resistance continues to mount.
Pharmaceutical use only scratches the surface. In polymer chemistry, researchers rely on 2-thenoic acid and its derivatives to tweak materials for electronics, coatings, and adhesives. For instance, scientists can combine this tiny acid with other parts to make plastic films that respond to light or heat. These films end up in smart packaging or display technology. By including tricky elements like sulfur and nitrogen in five-membered rings, 2-thenoic acid serves as a tool for customizing surfaces. These changes aren’t just abstract—real products, from flexible screens to protective layers in devices, benefit from research that begins with such versatile chemicals.
Synthetic chemistry would feel limited without access to molecular tools like 2-thenoic acid. Some chemical reactions hinge on its distinctive structure; the thiazole ring (a sulfur and nitrogen atom in a five-membered ring) provides a rigid backbone and special chemical behavior. This helps in forming bonds that would otherwise be difficult or less selective. For example, the acid group lets chemists make esters, amides, and other building blocks vital for research or special products. As a student, I remember how these reactions let my group create compounds that either lit up in tests or bound to specific proteins in disease studies.
Toxicity and environmental impact can’t be ignored. Like many laboratory chemicals, 2-thenoic acid requires careful handling. Exposure brings risks to people and aquatic life down the line. Making large quantities needs energy, and some methods create pollution. By backing greener chemistry initiatives, manufacturers and scientists constantly look for ways to limit waste and recycle solvents. Some teams pursue enzyme-driven methods to produce thiazole compounds more cleanly, hoping to support both progress and the planet. Research funding that encourages sustainable methods gives new life to chemicals like 2-thenoic acid, showing that small molecules can have a big impact when handled wisely.
2-Thenoic acid, a compound with a distinct character, shows up as a key player across multiple scientific fields. Also called thiophene-2-carboxylic acid, this chemical wears a tart aroma that resembles sour or smoky notes. It carries the formula C5H4O2S, bringing together elements that each pull their own weight.
Anyone who handles it typically finds it as a pale, off-white to beige crystal or powder. It doesn’t draw the eye with any flashy color, but the scent can’t be ignored. It melts at about 140°C, so it stands up to a fair bit of heat before changing form. The pro-quality here: that melting point makes it manageable in a lab, since you can work close to that temperature without vapor pouring off everywhere.
Solubility tells another part of the story. It blends pretty well with water, which isn’t always true for other aromatic acids. This feature helps researchers mix it in both organic and aqueous experiments. If you try to dissolve it in ethanol or ether, it gets along fine there too – a big plus for anyone experimenting with extractions or reactions.
Take a close look at its structure: the thiophene ring, a five-member ring with one sulfur atom, sets the stage. Attached at the two-spot, the carboxylic acid group adds acidity and opens doors to more reactions. This combination balances the aromatic stability of thiophene with the reactivity of carboxylic acids.
Acid dissociation constant (pKa) clocks in around 3.5, similar to benzoic acid. That matters for those working with buffers or needing selective acid strength. Its chemistry steps up when reacting with bases, forming water-soluble salts, and it acts as a moderate acid. In practice, it often gets used to build more complex molecules – especially where that sulfur atom and aromatic ring pull in specific electron behaviors.
Chemists reach for 2-thenoic acid when they need a starting point for medicines or new polymers. It gives medicinal chemists the backbone for many drug ideas where tweaking the carboxyl or the sulfur ring changes how the molecule interacts with the body. It shows promise in anti-inflammatory work, antibacterial studies, and as a marker for metabolic tracing.
Over the past years in the lab, the sulfur content in thiophene rings has added a new layer of intrigue for my colleagues working in organic synthesis. Unlike simpler aromatic acids, 2-thenoic acid participates in reactions where the ring can get modified or fused with other groups, without losing structure or purpose.
On the shelf, 2-thenoic acid doesn’t spoil easily. It stays solid under normal room conditions and resists breaking down under mild light or oxygen exposure. That shelf-stable nature makes storage simple; standard air-tight containers do the job. Take care, though, not to inhale its dust or let it near open flames, as fine particles can irritate or even ignite.
Making 2-thenoic acid sometimes involves steps that use harsh chemicals or generate waste. Green chemistry initiatives focus on catalysts and milder reagents to boost yield and lower environmental costs. If labs can adopt these improvements, costs drop, and safety rises – a win for everyone using it.
Whether for new antibiotics, electronic materials, or exploration of sulfur chemistry, 2-thenoic acid has carved out a solid place in science. Its dependable melting point, straightforward solubility, and sturdy chemical structure make it a tool that practical researchers rely on.
If you work around chemicals, it doesn’t take long to realize that every bottle and drum comes with its own personality. 2-Thenoic acid grabs attention with its sharp odor and distinct chemical nature. It’s used a lot in making medicines and specialty chemicals—so it shows up in more places than you might expect. Like any substance with bite, it rewards close attention to safety. Every good lab, shop, or warehouse aims to protect people and the environment, not just follow rules for the sake of it. My own experience has taught me to never underestimate what a splash or even a wisp of a strong-smelling powder can do if handled carelessly.
Every shelf, cabinet, or storage room tells a story about the work culture. Keeping 2-thenoic acid safe starts with containment. The acid likes to react with bases and strong oxidizers—so keeping it in tightly sealed glass or high-quality plastic containers helps prevent it from finding trouble. I’ve worked in places where someone reused an old soda bottle, only to end up with a warped mess and sticky residue. You avoid that pitfall with proper containers, distinct labels, and clear hazard symbols. Storage isn’t just about putting things away; it’s paying attention to what’s nearby. Every busy storeroom tries to stash as much as possible, but 2-thenoic acid doesn’t belong with bleach, ammonia, or anything flammable. Segregation is easy to overlook in a rush, but a minor slip can become a headache.
Heat brings out the worst in many chemicals. 2-Thenoic acid holds up best at room temperature—nothing too hot, nothing too cold, no wild swings. Too much humidity encourages clumping and can even start slow reactions that happen out of sight. My early days in research taught me to never trust a stuffy closet with zero airflow. Chemical vapor builds up, and pretty soon the space feels tight and harsh. Good ventilation makes a difference, even for a small stock. A fan and a functioning vent hood act as silent guardians against trouble.
Personal protective equipment is more than a checklist. A real pair of goggles, sturdy gloves, and even a dust mask set the difference between a normal day and an urgent shower run. No one looks cool in oversized gloves, but itchy hands tell a bigger story. Spills don’t care about confidence. If even a few grams hit the bench, clean-up kits, neutralizing agents (like sodium bicarbonate), and proper disposal routines save headaches and paperwork. Training everyone who crosses into chemical storage isn’t bureaucracy—it’s a way to head off the domino effect from one bad move. People learn quickly from mistakes, but a strong orientation saves scars and missed workdays.
It pays to keep your safety data sheets nearby. I’ve reached for them in the middle of tense moments more than once. Regular walk-throughs help spot cracked lids, damaged labels, or bottles left open. Good records on who takes what, and regular restocking of safety supplies, go farther than many folks expect. If there’s a spill, reporting it right away keeps the memory fresh—and helps improve the system for next time. Smart storage and mindful handling build a shop where people look out for each other, and every chemical stays in its lane.
Staying safe around 2-thenoic acid isn’t about paranoia—it’s about respect for the tools of our trade and the health of everyone who shares the workspace.Chemistry fills our shelves and workplaces with many names, some that sound more threatening than others. 2-Thenoic acid crops up in labs and certain industries, usually because of its role in chemical synthesis. People often ask whether this compound brings any real danger to those working near it or those using products created from it.
2-Thenoic acid falls into the category of organic sulfur compounds. Direct information about it can feel spotty, but a scan of toxicology sources, coupled with safety data sheets, starts to paint a picture. The Globally Harmonized System labels it as "irritating to eyes, skin, and respiratory tract." This means if someone breathes its dust or splashes it, they might deal with redness or coughing—not an immediate grave threat, but nothing to brush off either.
Animal studies haven't flagged major long-term issues at reasonable exposure levels, but there’s a lack of blunt-force toxicity data from human studies. Research has yet to tie 2-thenoic acid to cancer or genetic damage, so it doesn’t show up on lists of established carcinogens or reproductive hazards. At the same time, science only knows as much as it’s currently checked for, and a door to new findings always stays open.
In my experience, even a “mild irritant” gets respect in a lab. That trust in gloves, eye protection, and fume hoods comes not just from rules but from memory—colleagues who ignored steps and landed with rashes or watery eyes. Nobody wants a chemical burn under their wedding ring. Toxicity also doesn’t always shout its presence; the slow creep of chronic exposure can catch folks off guard. So, a bottle marked “irritant” stays behind a cabinet and gets routine checks to make sure it hasn’t leaked.
From a safety perspective, the best labs use substitution and minimization whenever a process allows—if less hazardous reagents deliver similar results, that’s the top pick. If not, the focus moves to risk controls. That might seem tedious, but every routine starts with a mishap somewhere else.
Exposure matters most. If 2-thenoic acid lands on the skin, prompt washing avoids most issues. If it gets in the eyes, plenty of water helps avert lasting harm. When heating or handling larger quantities, workers in regulated spaces use local exhaust ventilation. Most communities don’t face risks from this compound because it rarely sits in consumer products or soil.
Disposal practices carry their own weight. Pouring chemicals like this down the drain—a story I’ve heard too often—invites problems for water treatment and the wild mix downstream. Chemistry teachers and waste handlers often serve as the last line of defense against routine disposal mistakes.
The best step anyone can take is respect: read the label, use what science recommends, and keep usage minimal. Government agencies, universities, and chemical suppliers have steadily built out resources over the years so folks don’t need to rely on guesswork or out-of-date habits. That doesn’t make chemicals like 2-thenoic acid risk-free, but it pools our shared knowledge to prevent unnecessary harm.
The story of 2-thenoic acid echoes in many corners of chemistry—hazardous or not, stewardship really comes down to informed actions and vigilance. Each step builds on the last, always open to updating as science fills in new blanks. That’s the real safeguard and, in my experience, a source of steady confidence.
I’ve seen more researchers and DIY chemists searching for 2-Thenoic Acid in the past few months. A sulfur-containing compound tied to pharmaceuticals and certain dyes, it’s not like picking up paracetamol at the corner shop. The straightforward reality: distribution is tightly regulated thanks to its role in synthesis pathways for more sensitive chemicals. Online marketplaces such as Sigma-Aldrich, Alfa Aesar, and TCI Chemicals show listings for it, but nobody just lets you “add to cart” and pay with a credit card. Suppliers want to know who’s buying, and for what purpose.
Buyers from universities or licensed labs submit paperwork—company affiliation, project details, possibly import permits if outside the US or EU. For individual consumers, legal access is nearly out of reach. In my experience, the hoops aren’t just for show. These limits shield communities from accidental exposure and help authorities track high-risk substances.
Price tags jump around based on purity, order size, and region. Reliable suppliers list it anywhere from $250 to $600 per 25 grams, reflecting both production complexity and regulatory hurdles. Large-quantity buyers, such as pharmaceutical manufacturers, negotiate contracts directly. Pricing for small research labs or teaching collections rarely dips beneath $200 for small vials, if available at all. Some try lab supply portals out of Asia for cheaper prices, but quality and authenticity sometimes fall short—something I’ve had colleagues warn about in group chats.
Customs tariffs, VAT, and hazardous material fees stack up for global buyers. The invoice never looks like the upfront price. If your budget matters, this class of specialty chemicals may not be feasible outside sanctioned research.
Manufacturing and shipping 2-Thenoic Acid brings risks. Inhalation or skin contact can trigger serious reactions, and accidental releases during transport spark headaches for logistic teams. Reputable vendors invest in secure packaging and track deliveries like gold. I’ve seen controlled substances list similar requirements—special paperwork, locked storage, safety training for handlers. Mishandling the order doesn’t just risk fines, but also damages credibility in academic circles.
Some researchers consider making the acid from thiophene carboxylate precursors if procurement stalls. That route needs experienced chemists, full lab infrastructure, and local permits. Costs might drop, but the learning curve and safety risks often outweigh any savings, especially in smaller facilities or teaching labs.
For non-lab users or curious hobbyists, there’s no legal gray zone to exploit. Most attempts at bypassing suppliers or using chemical forums for black-market leads open buyers to criminal charges. Even for straightforward scientific investigation, transparency counts. Choosing reputable vendors, documenting purchases, and following reporting guidelines not only keeps teams safe, but supports open science and discourages shadow trades that threaten public trust.
Ethical procurement takes patience and planning. Before placing an order, teams should evaluate whether alternatives can achieve the same research aims, consider local partnerships (such as university core facilities), and budget for real-world chemical expenses. Open dialogue with institutional safety officers helps smooth the supply process and prevents disruptions. I’ve seen collaborations thrive when everyone pools knowledge and divides sourcing challenges. For newcomers, guidance from more experienced colleagues saves time and sidesteps unnecessary risks.
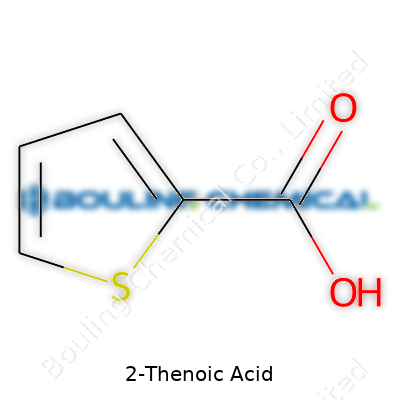
| Names | |
| Preferred IUPAC name | Thiophene-2-carboxylic acid |
| Other names |
Thiophenecarboxylic acid 2-Thiophenecarboxylic acid Thiophene-2-carboxylic acid |
| Pronunciation | /θiːˈnoʊ.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 147-93-5 |
| 3D model (JSmol) | `3DModel: JSmol/CC1=CSC2=CC=CC=C21` |
| Beilstein Reference | 1207330 |
| ChEBI | CHEBI:27260 |
| ChEMBL | CHEMBL2111197 |
| ChemSpider | 10979 |
| DrugBank | DB04318 |
| ECHA InfoCard | 100.011.702 |
| EC Number | 2.3.1.39 |
| Gmelin Reference | 6075 |
| KEGG | C01748 |
| MeSH | D013837 |
| PubChem CID | 70075 |
| RTECS number | XR0175000 |
| UNII | 4T8E370R3S |
| UN number | UN3261 |
| Properties | |
| Chemical formula | C4H4O2S |
| Molar mass | 157.17 g/mol |
| Appearance | White to light yellow crystalline powder |
| Odor | Odorless |
| Density | 1.432 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.99 |
| Vapor pressure | 0.01 mmHg (20°C) |
| Acidity (pKa) | 3.50 |
| Basicity (pKb) | 11.11 |
| Magnetic susceptibility (χ) | -68.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.595 |
| Viscosity | 1.219 mPa·s (25 °C) |
| Dipole moment | 1.613 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 186.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -212.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -520.7 kJ/mol |
| Pharmacology | |
| ATC code | N02AE02 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P261, P264, P271, P272, P280, P301+P312, P302+P352, P305+P351+P338, P362+P364, P330, P501 |
| NFPA 704 (fire diamond) | 2-Thenoic Acid NFPA 704: 2-1-0 |
| Flash point | Flash point: 113°C |
| Autoignition temperature | 285 °C |
| Lethal dose or concentration | LD50 (oral, rat): 640 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 790 mg/kg |
| NIOSH | TH0700000 |
| PEL (Permissible) | PEL not established |
| REL (Recommended) | 'COOH2T-01' |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Thiophene 3-Thiopheneacetic acid 3-Thiophenecarboxylic acid Furan-2-carboxylic acid Pyrrole-2-carboxylic acid |