Back in the early twentieth century, chemists searched for better solvents and building blocks for polymers. In this quest, they uncovered 2-pyrrolidone. Its discovery linked up with the work on derivatives of γ-aminobutyric acid in the 1930s. Industrial interest followed quickly, especially as nylon's popularity spread. This material emerged at a time when demand for high-performance chemicals was climbing, driven by the textile and pharmaceutical sectors. Synthetic routes matured, and by the 1950s, entire plants produced 2-pyrrolidone to supply polymer industries. Growth of nylon production and the development of pharmaceuticals fuelled further research and commercialization. This molecule now plays a role in many fields, thanks to decades of targeted innovations.
2-Pyrrolidone holds a solid spot as a colorless to pale yellow liquid at room temperature. Manufacturers value it for its role as a versatile chemical intermediate, an active solvent, and a base for making medicines and polymers. Integration into different industries stems from its solubility in water and organic solvents, as well as a strong ability to break down resins, plastics, and rubber. Chemical suppliers package it for laboratory use, pharmaceutical synthesis, and industrial processing, with specifications aimed at purity and concentration according to the end-use. Raw material suppliers satisfy a spectrum of needs, from small-batch labs to high-volume production sites.
Looking at its characteristics, 2-pyrrolidone shows a boiling point of about 245°C and a melting point close to 25°C. Its molecular formula is C4H7NO, and it weighs in with a molar mass of about 85.1 g/mol. Miscible in water, alcohol, acetone, and ether, this compound delivers a powerful solvent capacity with a high dielectric constant. It remains relatively stable under ambient conditions but reacts with strong oxidizers. Its viscosity matches lubricants and aids in applications from inks to specialty coatings. Even after decades, manufacturers depend on its robust and predictable chemical profile.
On the factory floor or in a laboratory, users expect certain benchmarks for this chemical. Big suppliers usually deliver it at purity levels ranging from 99% to 99.5%. Water content often needs to stay below 0.5% by mass. Impurities, such as gamma-butyrolactone, sit well under 0.1%. Product drums bear hazard identifications, batch numbers, and shelf-life dates, together with transportation classifications under GHS and IMDG codes. Labels set forth safety guidance, chemical structure, and regulatory statements about handling and storage. Auditors check compliance by verifying technical data sheets and certificates of analysis with every lot.
Large-scale factories usually begin synthesis by reacting gamma-butyrolactone with ammonia or by cyclizing 4-aminobutyric acid. These routes allow for relatively clean conversion at industrial volumes. Key steps revolve around precise control of temperature, pressure, and catalyst. Many facilities rely on batch reactors for modest volumes, while continuous processes serve high-throughput operations. After synthesis, purification steps pull out side-products, water, and any unreacted substrate. Advances in catalysis and separation have made it possible to reach impressive purities while managing costs.
2-Pyrrolidone can react further to create a range of derivatives. Alkylation produces N-methyl-2-pyrrolidone (NMP), another major industrial solvent. Hydrolysis under acidic or basic conditions yields gamma-aminobutyric acid, widely used in medicine. Serving as a precursor, it allows for ring-opening reactions and serves as a source of nitrogen in heterocyclic synthesis. Catalytic hydrogenation leads to pyrrolidine, an important building block. Functionalization at the nitrogen atom tailors its properties for polymerization or for drugs targeting neurological pathways. In industry, operators take care to avoid mixes that trigger unwanted polymerization or decomposition.
2-Pyrrolidone bears several names in the scientific literature and product catalogs. Old-school chemists still call it 2-pyrrolidinone. Across global markets, it turns up as γ-aminobutyric acid lactam, 1,2-pyrrolidone, and pyrrolidin-2-one. Some suppliers label it “2-oxo-pyrrolidine,” especially when emphasizing its use as a lactam. Others, especially in pharma, refer to it in shorthand as “2-Py” or “PLD.” Checking these names against regulatory databases helps users confirm identity and application approvals.
Working with 2-pyrrolidone calls for respect and careful standard practice. Splash contact can irritate eyes or skin, so operators wear gloves, lab coats, and protective eyewear. Prolonged inhalation of vapors in enclosed spaces can harm the respiratory tract; good ventilation and fume extraction stay top priorities. Storage guidelines recommend sealed, inert containers kept in cool, dry rooms away from oxidizing agents. Chemical safety data sheets back up site-specific protocols. Emergency response teams keep spill kits and cleanup procedures on hand, trained to handle leaks or accidental exposures. Lab and production managers schedule regular safety drills, reinforcing the importance of vigilance in daily practice.
2-Pyrrolidone has found its way across industries. It serves as a solvent for polymers, resins, and dyes, making it a staple in factories producing specialty inks and coatings. In pharmaceuticals, it acts as a precursor for anticonvulsant drugs, cognition enhancers, and other medicines targeting the central nervous system. The agrochemical industry uses it to synthesize herbicides and plant protection agents. Engineers add it to electrolyte solutions in lithium batteries, drawn by its conductivity and thermal stability. Its chemistry propels research in biodegradable plastics and advanced materials. It even interfaces with the world of adhesives, where its liquid properties boost performance in challenging environments.
Chemists across universities and industry invest in tweaking and expanding the uses of 2-pyrrolidone. Research teams work on more sustainable synthetic routes, aiming to lower waste and energy use. Specialists in drug discovery use its backbone to build new compounds for neurological, metabolic, and oncological diseases. Materials scientists customize it for high-strength polymers, new battery chemistries, and conductive inks. Manufacturers watch for tighter purity thresholds and better recycling methods. Modern laboratories run comparisons with other lactams to uncover unique reaction pathways. Academic groups publish on functionalization and ring modifications, opening the door to next-generation products.
Toxicologists have spent years mapping out the risks and safe exposure limits of 2-pyrrolidone. At high concentrations, lab animals show respiratory, neurological, and developmental effects. Smaller, chronic exposures tend to focus on skin and eye irritation or mild central nervous system symptoms. Data shows moderate acute oral toxicity. Agencies such as the US EPA and European Chemicals Agency review and update occupational exposure limits for workers. Regulatory science continues to fill knowledge gaps around chronic exposure, reproductive risks, and potential environmental impact. Research pushes for greener manufacturing processes and lower-toxicity derivatives to cut risks for both users and handlers.
Looking at the future, 2-pyrrolidone sits at an intersection of progress in pharmaceuticals, polymers, and electronics. Companies seek to cut environmental footprints, so they invest in cleaner synthesis and recycling. Interest in biodegradable plastics and alternative solvents grows as legislation tightens worldwide. The boom in battery-powered devices and electric vehicles stirs demand for specialty electrolytes, often built on this molecule’s core structure. Some researchers eye the ring-opening reactions for tailoring bioactive compounds, while others link it to next-generation fibers and performance coatings. Sustainable sourcing and process intensification remain hot topics, with both academic and industrial teams competing to make every gram count, not only for cost but for health and environmental safety.
2-Pyrrolidone might sound like something that only belongs in a chemistry lab, but its reach extends well beyond scientific circles. This clear, nearly odorless liquid shows up in products found in offices, homes, and even farms. I’ve seen it most often as a silent partner, quietly doing the heavy lifting in industries that focus on convenience and efficiency.
Inkjet ink relies on 2-Pyrrolidone to keep the color flowing smoothly. As someone who worked my share of late-night print jobs, nothing frustrates quite like streaky or dried-up prints. Formulators turn to 2-Pyrrolidone because it dissolves pigments and other ingredients, making the ink stable and reliable. End users get bolder colors and longer shelf life, and print shops cut back on wasted supplies.
Some companies turn to this liquid for pharmaceutical production, particularly for medicines that require precision in their formation. You’ll find it acting as a solvent in injectable drugs, topical medications, and oral tablets. US regulators like the FDA keep a close watch on every solvent used in medicine; 2-Pyrrolidone counts as safe for certain applications, based on strong evidence about its effects at medically approved doses. As with anything synthetic, the people who make the products have to communicate exactly how much is present for those who need to monitor health concerns.
Cleaners and surface strippers often use this ingredient. From scrubbing grime off engine parts in auto garages to prepping plastic before painting, 2-Pyrrolidone gets the job done. My own experience with household paint removers brings home how it breaks down stubborn coatings that nothing else seems to touch. Safety becomes important here; users need to wear gloves and work in well-ventilated areas, since skin contact can cause irritation.
Out on the farm, crop protection products sometimes include 2-Pyrrolidone for dissolving herbicides and finding the right balance with other chemicals. It helps keep formulas stable, especially for products that spend weeks on warehouse shelves before application. Looking at plastics, 2-Pyrrolidone forms part of the building blocks for high-performance nylon. This plastic shows up in car parts, sports equipment, and clothing fibers known for lasting longer and standing up to heavy use.
No useful chemical comes without its share of concerns. Studies point out that skin or eye exposure can cause discomfort, and swallowing or breathing in high amounts can lead to health issues. At environmental levels used in household products, experts say risks are low with normal precautions. Still, there’s always a conversation about keeping chemical use as safe and transparent as possible. Companies can provide better labels and instructions for handling. Workers can benefit from solid training, and manufacturers can keep researching greener, safer alternatives for sensitive settings.
Change rarely comes easy in industrial chemistry, but open dialogue about safety and environmental impact leads to progress. Whether creating the ink for our morning reports or the plastic parts in reliable vehicles, 2-Pyrrolidone stands as one of those behind-the-scenes helpers. As researchers test newer options and regulators keep watch, industries can look forward to products that balance performance and responsibility. For people who touch these products at home or work, clear information and basic safety steps go a long way toward keeping things worry-free.
2-Pyrrolidone sounds intimidating at first glance, but plenty of folks walk past or even work with it every day. It shows up in things like inkjet printer ink, pharmaceuticals, adhesives, and cleaners. Some might even know it from the world of nylon production. With chemical names so long, the real worry rests on what happens if your skin, lungs, or eyes cross paths with it.
Fact is, 2-Pyrrolidone doesn’t act like the most aggressive industrial compounds. Touch it, and it might cause a little redness, especially for people with skin on the sensitive side. Toss a splash in your eye, and you’ll wish you wore glasses that day. Its vapor, though, stays low in most open, ventilated spaces—most won’t even notice the odor unless the stuff’s concentrated. If you spill it, the briefest contact doesn’t threaten your health. The trouble comes with repeated or heavy exposures, where it can irritate eyes, skin, and respiratory passages. No one wants to inhale anything they can’t pronounce, but 2-Pyrrolidone does not build up in the body and isn’t classed as a cancer risk by organizations like the International Agency for Research on Cancer.
I’ve worked with solvents and industrial chemicals for years, and I always look up the latest Safety Data Sheets before opening a drum. For 2-Pyrrolidone, those sheets point to the same cautions you’d see with strong household cleaners. Gloves, goggles, and a window cracked open or a fan running do the trick. Workers in manufacturing put on more gear and make sure spills don’t linger. While the stuff shouldn’t go on the skin routinely, most exposures won’t spark a trip to the doctor unless someone ignores basic rules.
Waste remains one area where care matters. Pouring it down the drain can trouble local water supplies, not just plants and fish but also the people downstream. That’s why industry captures spills and routes waste for special handling. European and U.S. regulatory agencies set workplace exposure limits—so companies stick to tighter controls in larger operations. Since it’s biodegradable, natural processes usually break it down in the environment, but large releases or mismanaged waste can still harm wildlife.
Safety always improves with training and the right gear. Reading even a brief fact sheet or catching a training video before opening a bottle helps. At home, even a splash of household cleaner or irritant should get rinsed off right away. If workplaces invest in better ventilation, keep safety showers handy, and store containers securely, the risk dips lower.
Even as new alternatives hit the market, industries stick with 2-Pyrrolidone because it works. With smart handling, the risk doesn’t loom large for most people, but no one should take chances with their health—especially if they work with chemicals every day.
2-Pyrrolidone shapes a lot of what we see in pharmaceutical chemistry and industrial production. This chemical carries the formula C4H7NO. Looking at the name, 2-Pyrrolidone, there’s a five-membered lactam ring in play—basically, the structure includes four carbon atoms, one nitrogen atom, and one oxygen atom. The oxygen comes from a carbonyl group, sitting right next to the nitrogen in the ring, making it a cyclic amide. Chemists sketch it out as a pentagon with a double-bonded oxygen off one of the corners, and the nitrogen tucked into the ring.
Anyone who has dipped a toe in polymer production or drug formulation has probably worked with this compound or relied on something made from it. 2-Pyrrolidone goes way beyond the lab bench; it serves as a building block for N-vinylpyrrolidone, which leads to polyvinylpyrrolidone (PVP). Walk into any pharmacy, pick up a bottle of iodine disinfectant, and there’s PVP iodine, making sure wounds stay clean.
The reason 2-Pyrrolidone matters stems from its versatility. This chemical shows up not just in medical products but also in the manufacture of solvents, inks, and even herbicides. Back in graduate school, I remember it as one of those go-to compounds for dissolving polymers that never seemed to budge in anything else. Its structure, with both a polar amide group and a non-polar backbone, lets it mix with water as well as organic chemicals. That’s real flexibility.
A compound like this doesn’t land on a factory floor or in a pill bottle without meeting tough regulatory standards. Every time industries work with 2-Pyrrolidone, its chemical structure matters for handling and safety protocols. The carbonyl group makes it a bit reactive; industrial chemists need to watch out for conditions that might lead to polymerization or unwanted side reactions. I’ve seen spills in the lab where that slightly amine-like odor hints at its presence, reminding everyone to check the material safety data sheet before cleaning up.
Factoring in occupational exposure matters too. OSHA and other safety bodies draw lines for permissible exposure levels based on data from real workers. That kind of oversight doesn’t happen by accident; it’s built on a foundation of chemical understanding, practical experience, and years of accumulated lab data.
Solutions for safe handling are about more than goggles and lab coats. Facilities can invest in closed-system transfers, proper ventilation, and robust chemical inventory tracking—steps that grow out of recognizing the molecular structure and its risks. Waste streams containing 2-Pyrrolidone need responsible management because water treatment plants don’t always break down amide-based compounds fully. Incineration at controlled temperatures works better, cutting down on residue and secondary contamination.
Training workers and students alike in the chemistry—and not just the rules—leads to smarter, safer labs and factories. The more hands-on experience with compounds like 2-Pyrrolidone, the quicker teams can spot anything unusual and prevent problems before they snowball.
The structure and formula of 2-Pyrrolidone open up a world of useful applications, but the story goes deeper than a list of uses. A solid grasp of its chemistry and the practical challenges it brings supports not only better science but also clearer paths to a safer workplace and healthier environment.
Anyone who’s been around a lab or chemical warehouse long enough knows chemicals like 2-Pyrrolidone deserve respect. It’s a handy solvent found in everything from medication to paint removers, but there’s a catch: getting sloppy with storage or handling creates problems. I’ve seen containers start to leak or corrode just because someone cut corners on proper sealing or used the wrong material. Problems often start simple and snowball fast.
2-Pyrrolidone doesn’t look scary at first glance. It’s usually a colorless liquid, has a faint odor, and feels pretty unremarkable. Yet, the fact remains—it can irritate eyes, skin, or even your lungs if you breathe in too much vapor. That’s why good storage trumps taking risks every single day.
In practice, that means using containers made from materials that don’t react with this chemical. I lean toward high-quality plastic or stainless steel. Don’t use rusty drums or anything with mystery coatings. Keep the lids tight, not just to prevent spills, but to slow down evaporation. A dry, cool storage spot makes a big difference. I’ve seen products go from shelf-stable to dangerous just from being stashed near a hot motor or sunlight.
One thing I never forget—ventilation. Storing 2-Pyrrolidone in cramped, stuffy spaces raises the stakes. If a leak happens, fumes spread rapidly, turning a headache into an emergency. Installing a good exhaust fan or at least storing chemicals in a well-aired room pays off—both for health and to stay clear of legal trouble.
Splash goggles, gloves made for chemical work, and a long-sleeved lab coat are a must. It’s not overkill. I’ve seen enough folks shrug off PPE and end up with red eyes or chemical burns. Wash up before you eat or use your phone. Chemical residues linger on fingers, and accidents happen quicker than most realize.
Pour slowly and use funnels that fit the container. Spills are easier to clean up on the bench than once they run under equipment. If something does spill, I grab absorbent pads made for solvents and clean up right away. Toss the waste into the correct disposal drum, never down the drain. Cleanup gets tricky in busy workspaces, but pretending everything is fine leaves long-term headaches—both chemically and from regulatory watchdogs.
Weekly drills and safety briefings might feel like a chore, but they build habits. Nobody wants a full-on hazmat response just because a tech didn’t know what to do. Sharing practical stories keeps folks alert. I remember a colleague storing 2-Pyrrolidone in a glass bottle, stashed near window light. After a few hot summer days, the bottle cracked, soaking a shelf. That incident got everyone serious about upgraded storage and labeling. Mistakes in one spot teach everyone else a lesson—if the story gets told.
Most problems never make it to the news. That’s because ordinary, routine safety keeps them in check. Cut costs or get lazy, and the odds catch up. Simple measures—double-checking labels, keeping up with maintenance, giving workers real training—make all the difference. No fancy solution needed, just common sense and a refusal to treat chemicals like they're as harmless as water.
People often ask about purity and grade whenever they source chemicals like 2-Pyrrolidone. That question carries real weight. Purity isn’t just a number—it’s the straight answer to what’s in your bottle and what isn’t. If you’re running a research project, making pharmaceuticals, or working on polymers, impurities could throw off your results, safety, or product function.
In my work with specialty chemicals, I’ve seen how low-purity grades end up causing more problems than they solve. Imagine spending months troubleshooting a batch only to realize the issue came from slight contamination in the raw materials. With 2-Pyrrolidone, tiny shifts in composition can affect how the material behaves in solvents, how it reacts with other compounds, or whether you hit your quality targets in final products.
High-grade 2-Pyrrolidone for pharmaceutical and electronic industries usually clocks in at 99.5% or higher. Grades meant for less sensitive work, like industrial coatings or ink, typically come in at about 99%. Anything lower than this probably wouldn’t work for making fine chemicals or medicines. It might pass for simple cleaning agents, but not much else.
You want certificates that back those numbers. Reputable suppliers hand over a Certificate of Analysis on every lot. That sheet lists the breakdown of water content, color, and known impurities, like ammonia or heavy metals. One bad batch could mean lost time or compromised compliance, especially if you need to meet strict regulations.
Labels such as “pharma grade” or “technical grade” get thrown around. My approach: look past the name and ask what actually got tested. Pharma or reagent grade means the 2-Pyrrolidone passed checks for things like metal content, organic residues, and water. Lower grades, picked for price, often escape such scrutiny. They come from larger production runs, sometimes using older equipment or recycled feedstocks.
I once visited a plant where control slipped, and the whole line got traced back to improper storage conditions. Such situations prove why supply chain transparency matters. Reliable producers don’t just list a purity figure; they invite audits, publish lab results, and respond directly to customer questions. Good companies track every detail, from raw material origin to logistics.
My advice: ask for batch-level traceability and third-party analysis. Cross-check listed specs with recognized pharmacopoeias (like USP or EP). If a vendor hesitates to share this, there’s usually a reason. Look for ISO-certified operations; they tend to follow strict handling rules and documentation requirements.
Some buyers chase the lowest offer. Cheaper 2-Pyrrolidone sometimes brings residues not listed in specs—odd odors, yellowing liquids, unpredictable viscosity. I’ve watched R&D teams lose whole development cycles to these “minor” surprises. Spending a little extra upfront beats wrestling with hidden setbacks that take months to fix.
Solid purchasing choices always focus on reliability, documentation, and the actual chemistry at hand. That’s how you protect your lab, your process, and, in many cases, your reputation down the line.
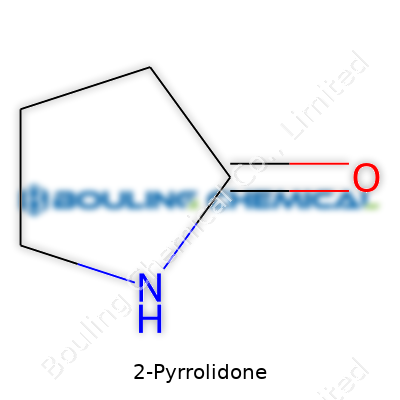
| Names | |
| Pronunciation | /paɪˈrɒlɪˌdoʊn/ |
| Identifiers | |
| CAS Number | 616-45-5 |
| 3D model (JSmol) | `3D;JSmol;C1CC(=O)NC1` |
| Beilstein Reference | 605603 |
| ChEBI | CHEBI:18049 |
| ChEMBL | CHEMBL961 |
| ChemSpider | 5589 |
| DrugBank | DB00189 |
| ECHA InfoCard | 100.019.114 |
| EC Number | 211-162-9 |
| Gmelin Reference | 7877 |
| KEGG | C00751 |
| MeSH | D011693 |
| PubChem CID | 7021 |
| RTECS number | UY8725000 |
| UNII | 9M1UVD043J |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C4H7NO |
| Molar mass | 85.10 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Faint amine-like odor |
| Density | 1.116 g/cm³ |
| Solubility in water | Miscible |
| log P | -0.85 |
| Vapor pressure | 0.016 hPa (20 °C) |
| Acidity (pKa) | 14.9 |
| Basicity (pKb) | -1.44 |
| Magnetic susceptibility (χ) | −8.0×10⁻⁷ cm³/mol |
| Refractive index (nD) | 1.483 |
| Viscosity | 47 mPa·s (25 °C) |
| Dipole moment | 4.09 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 116.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -504.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2325 kJ/mol |
| Pharmacology | |
| ATC code | D11AX10 |
| Hazards | |
| Main hazards | Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, Warning |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 154°C |
| Autoignition temperature | 315 °C |
| Explosive limits | Explosive limits: 2.3–31% |
| Lethal dose or concentration | LD50 Oral Rat 6500 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 6500 mg/kg |
| NIOSH | RN 872-50-4 |
| PEL (Permissible) | PEL not established |
| REL (Recommended) | 100.00% |
| IDLH (Immediate danger) | 100 ppm |
| Related compounds | |
| Related compounds |
Pyrrolidine N-Methyl-2-pyrrolidone Succinimide Butyrolactone Proline |