Chemists first explored the imidazole family well over a century back, chasing after nitrogen-based rings with handy uses in medicine and chemistry. As science pressed on, folks realized not every imidazole acts the same. The addition of a propyl group on the 2-position created a whole new flavor: 2-propylimidazole. From the 1970s onward, industry discovered that this tweak handed manufacturers a sturdy and versatile intermediate, not just for pharmaceuticals but for sophisticated coatings and polymer work. Anyone with a background in organic synthesis knows how often small changes flip the table in reactivity, and the propyl tail certainly did the trick.
2-Propylimidazole stakes out its territory as both a base building block and a specialty chemical. You find it most often as a reagent in labs or as a component of specific catalyst blends. Some companies bottle it as a pure solid, while others fold it into custom chemical cocktails. Users caring about purity, batch consistency, solvent residue, and even packaging type soon discover that not all suppliers treat it the same way. Anyone in charge of quality control feels the push and pull between price and grade the moment this compound shows up on a product list.
In its pure, crystalline form, 2-propylimidazole typically appears as a white to pale off-white solid. Its melting point floats around 62-66 °C, putting it right in the sweet spot for ease of handling in labs and pilot plants. Vapor pressure stays low at room temperature; you won’t catch it evaporating on the bench during routine syntheses. As for solubility, it stirs in nicely with most polar organic solvents, such as methanol and ethanol, but it prefers not to mix with plain water. Chemists notice its sharp, pungent odor the moment a bottle is cracked. Its molecular formula is C6H10N2, clocking in at a molar mass of 110.16 g/mol.
Buyers constantly scan for clear technical sheets. On the label, major suppliers display purity in percentage, often above 98%. Moisture content, color index, and residual solvents all show up if you dig deep enough into results from the quality lab. Pack sizes range from tiny glass ampoules for research to bulk plastic drums for manufacturing. UN numbers flag safe shipping. Labels, in my own experience, sometimes leave out storage recommendations—something safety managers can’t ignore, especially where temperature swings might degrade the product.
Traditional synthesis starts from imidazole itself. Most routes rely on N-alkylation. You simply treat imidazole with a propyl halide (such as 1-bromopropane or 1-chloropropane) in the presence of a strong base. Potassium carbonate and DMF as the solvent get the job finished for most benchtop-scale work; for industry, continuous feed reactors and carefully-tuned mixing devices keep yields up and waste down. After the reaction, purification runs through distillation or recrystallization, washing away byproducts like unreacted starting materials and excess base. Nowadays, people tinker with greener solvents and flow chemistry to scale production without leaving a heavy environmental footprint.
2-Propylimidazole reacts as both a nucleophile and a base thanks to its nitrogen-rich core. It slips neatly into alkylation and acylation reactions, and the propyl group prevents unwanted ring substitutions during follow-up chemistry. In coordination chemistry, the compound forms complexes with a variety of metal ions, making it valuable in catalyst development. For folks working on polymers, 2-propylimidazole serves as a functional group to tether on chains, while in pharmaceuticals, it blends well with other heterocycles for experimental scaffolds. Anyone who's spent time in a synthetic lab knows, versatility always counts for plenty.
Across catalogs and journal articles, 2-propylimidazole goes by a few alternate identities. Some suppliers mark it as 2-n-propylimidazole, N-propylimidazole, or call it by its less common registry numbers: CAS 17413-50-2 or EINECS 241-429-7. Brand names rarely pop up, since it’s not sold in consumer-ready blends, but research and patent filings might wedge it into proprietary acronyms or code names as its application broadens.
Anyone handling this compound should pay attention to exposure limits. The solid and its vapors can irritate eyes, skin, and lungs. Proper lab coats, gloves, and eyewear ought to be the rule, not the exception. Fume hoods make sense for work with volatile solvents. Disposal procedures call for secure containers and bona fide chemical waste facilities—tossing this down the drain, even in dilute form, violates plenty of local codes. If someone lets a spill slide or skips the eyewash station, problems snowball fast. This isn’t a chemical for sloppy storage or lax housekeeping. Written safety data sheets map out emergency steps, but leadership makes the difference in whether those plans stay more than paper.
Over time, 2-propylimidazole found a home in several fields. The pharmaceutical labs look to it as a key intermediate, especially for antifungal and antiprotozoal drugs. Battery and electronics researchers put it to use in ionic liquids, looking for stable electrolytes. Polymer chemists value its ability to modify backbone structures, increasing strength or flexibility. Catalysis researchers know it can tune reactivity patterns in transition metal complexes. Some niche crops in agriculture tap it for experimental growth regulators. Curious technologists keep finding new corners where its nitrogen-rich structure fits.
Process optimization keeps fueling R&D efforts, including more efficient synthesis methods and cleaner purification workflows. I’ve watched colleagues chase after chiral variants and test modifications around the imidazole core for novel biological effects. Funding agencies encourage work toward greener chemistry, prompting work on continuous flow and bio-based starting materials. Scholarly journals regularly feature new uses, from advanced materials to environmental sensors. Real innovation takes time, but the trail keeps branching out as more folks turn their attention to underappreciated intermediates like this one.
Toxicologists track any new findings closely. 2-Propylimidazole, like many nitrogen heterocycles, shows low acute toxicity at the doses typically encountered in industry or research settings, but animal studies flag mild to moderate irritation, especially after skin or inhalation exposure. Chronic exposure data stay limited, which means regulatory caution gets built into safety protocols. No reliable evidence ties it to mutagenicity or carcinogenicity under proper handling, but folks dealing with kilogram lots treat any unknown with respect. Larger scale assessments need more robust environmental fate and breakdown studies, so environmental chemists won’t be leaving their monitoring kits at home any time soon.
Opportunities look bright for anyone staking a claim in specialty chemicals or advanced intermediates. The ongoing shift toward more efficient, sustainable reactions places 2-propylimidazole as a useful candidate for both large and small innovation teams. Its balance of stability and reactivity means it keeps catching attention, especially as new materials science and battery tech accelerate. The need for fresh ideas in medical chemistry and sensor tech hints that further modifications and hybrid derivatives may soon step into the spotlight. As research grows, regulatory clarity and smarter safety standards will come alongside, helping this simple compound keep evolving to meet changing industrial and research needs.
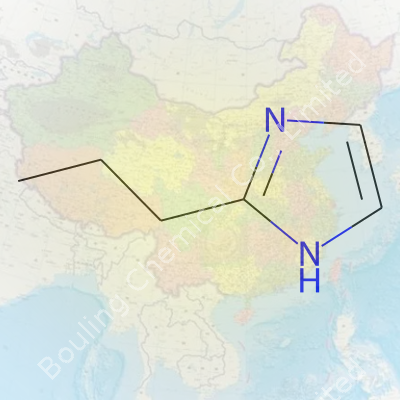
2-Propylimidazole doesn’t exactly roll off the tongue, and its chemical structure isn’t something you’ll see swirling in your morning coffee. To picture it, think of the imidazole ring, which looks like a five-membered ring containing two nitrogens at spots 1 and 3. Stick a propyl group—basically a chain of three carbon atoms—on the second carbon spot. That’s where the “2-propyl” part steps in. Written out, the molecular formula reads C6H10N2. People in the chemistry world write its structure as “C3H7–C3H3N2” to capture both the propyl chain and the imidazole core. For folks who like drawings, this means a propyl group hanging off an imidazole ring like a stubborn offshoot on a tree.
Chemical structures seem academic until you realize how much hinges on them. That ring system—two nitrogens placed just right—gives imidazoles a knack for hooking up with metals, building blocks in drugs, and shuttling protons in living systems. Tacking the propyl group on brings new action to the table. It tweaks how the molecule behaves—how it dissolves, how it binds to other chemicals, even which enzymes it attracts or annoys. This particular structure pops up in research circles hunting for new catalysts, new pharmaceuticals, and new materials. Some folks don’t blink an eye at fine chemical tweaks, but those little changes can make or break an experiment.
In my own college days, I spent late nights with sharpies and molecular model kits, learning that one swapped chain or ring wrecks a whole synthesis. Labmates and I struggled with the difference a propyl tail could bring. My roommate once swapped in an ethyl group where a propyl belonged, and the reaction stubbornly stalled. There’s a lesson sitting right there: details count. Medicinal chemists rely on small tweaks like this when turning a bland molecule into a drug that fights infections or controls blood pressure. Even a propyl boost can punch up drug absorption or cut out side effects. That’s the sort of impact you find only by looking closely at structure, not just at names or formulas.
2-Propylimidazole won’t make headlines itself, but the way it fits in molecules matters to several industries. Chemical factories synthesize these kinds of imidazole derivatives for custom catalysts. Drug hunters see N-alkyl imidazoles like this one as a bread-and-butter piece of their toolkit. One concrete challenge is the environmental impact—organonitrogens like this find ways into wastewater streams after use in factories. Treatment options exist, but they cost money and time. Green chemistry offers some hope for safer synthesis, using water as a solvent and milder conditions, so you get the same useful building block but cut down on toxic leftovers. Investing in these routes helps more than just chemists—it keeps chemicals like 2-Propylimidazole from becoming a future pollution headline.
2-Propylimidazole might sound like something tucked away on a dusty shelf in a chemistry department, but this compound finds its way into much more practical corners of modern life. Talk to folks in the coatings or polymer fields and the name comes up. The story gets interesting once you look at what this substance actually does, both in factories and in final products.
Anyone who’s worked with adhesives or paints has heard about epoxy resins. These resins need help “curing”—turning from a sticky mess to a solid, durable surface. 2-Propylimidazole steps in as a curing agent. The reason manufacturers keep picking it has to do with its knack for speeding up hardening, so factories can crank out goods at a steady pace instead of waiting around for hours on end.
Look at electronics. From circuit boards in smartphones to the power supply in laptops, the reliability of these devices depends on well-cured epoxies. A consistent, reliable hardener matters, because every poor cure risks faulty products and warranty headaches. 2-Propylimidazole carries its weight here by making sure the transition from liquid to solid doesn’t drag on or remain incomplete, which could spell disaster for tiny electrical connections.
I once helped a small machine shop pick new floor paint. The place ran twenty-four hours a day, and downtime wasn’t an option. The contractor insisted on a paint system using an imidazole-based hardener. Fast curing at room temperature cut hours off the drying time. Turned out, that choice traced back to 2-Propylimidazole. Not only does it help coatings set up faster, but it also aids in making finishes tougher and longer-lasting, able to stand up to heavy boots, oil spills, and dropped wrenches.
Polymer chemists chase after the right balance between processing and performance. 2-Propylimidazole shows up in specialty plastics and rubbers not just for curing, but for tweaking structure on the molecular level. By controlling the reaction speed and, in some cases, the density of cross-links in a polymer, this little molecule influences flexibility, chemical resistance, and even color stability.
Problems crop up if supply gets tight or prices jump. Some manufacturers might turn to other amine-based accelerators, but side reactions can bring unwanted odors or weaker end products. I’ve heard of batches ruined by hasty substitutions, which just adds more waste and costs. Sourcing a reliable supply boils things down to trust between suppliers and manufacturers—nobody wins if one piece of the chain falters.
Safety managers don’t ignore what’s coming in the door. 2-Propylimidazole, like many industrial chemicals, needs careful handling. Skin contact or inhaling fumes isn’t wise, and workers rely on gloves and ventilation. Pipelines and vats come with solid safety plans. It’s easy to overlook these requirements until someone gets careless, but better knowledge and training avoid problems. The industry keeps watch for safer alternatives, but as things stand, personal protection ranks as a first defense rather than a luxury.
In the right hands, 2-Propylimidazole keeps lines running and products strong. Better communication between users, suppliers, and safety experts leads to fewer headaches and safer workplaces. New research might uncover gentler substitutes or smarter processes, but for now, its role stays secure in plenty of workshops and labs across the globe.
Walk into any storeroom holding chemicals, and one thing stands out right away: safety comes first. With 2-Propylimidazole, that same mindset makes all the difference between a calm lab and one ticking toward trouble. This compound isn’t used in every high school classroom, but chemists working with it need to pay close attention. At room temperature it’s a colorless liquid or pale yellow, mild in appearance, but the story doesn’t end at how it looks in a bottle.
Here’s what’s at stake: 2-Propylimidazole catches fire more easily than water will ever put it out. Vapors can irritate skin, eyes, and lungs. Anyone who’s splashed a harsh substance on bare skin or caught a whiff of something strong in a fume hood knows discomfort comes quick. In daily life, we put lids back on paint thinner and lock up cleaning products under the sink—not out of paranoia, but from lessons learned after rashes or headaches. For chemical professionals, that instinct gets dialed up.
Cool, dry, and well-ventilated rooms—those matter more than fancy alarm systems. Moisture in the air or a hot storeroom can break down bottles or send fumes farther than they should go. Flammable liquids find their home in metal safety cabinets away from ignition sources. Shelves stay uncluttered so a glass bottle can’t tumble off the edge. Reading about controlled access may sound dry, but take it from someone who’s seen neglected storage rooms: one loose cap or corroded container can spell a ruined afternoon, or much worse.
Most places that deal with these materials have clear labeling. Personal experience showed me how critical this is—one mix-up, especially with something flammable, creates real panic. Color-coded tape, clear hazard pictograms, and legible writing beat even the fanciest digital inventory software any day if a new worker needs to make a split-second decision.
Before touching 2-Propylimidazole, gloves aren’t negotiable. I’ve worked with people who cut corners, and eventually they learn the hard way that a minor chemical burn can mess with your week. Splash goggles, long sleeves, and chemical aprons keep the odds of an ER visit down. Hoods or extraction fans should run every time—not just on inspection days.
Decanting into smaller bottles or syringes adds another layer of risk. Bulk storage may sound simpler, but smaller, dedicated containers reduce handling mistakes. Keep only the amount you need. If a bottle ends up with a corroded cap or if you notice any kind of strange color or weird smell, it’s smarter to dispose of it immediately, according to hazardous waste procedures you can trust—not out behind the dumpster.
I can still recall training days spent cleaning up after spills: the right absorbent material, the right container, no trickle-down the drain. Cleanup materials go right into hazardous waste bins, not any old trash can. Dry sand works better than traditional rags because it won’t react with the spilled liquid. Keeping a spill kit nearby isn’t just about following the rules, but about protecting everyone who comes in after you clock out. Most slip-ups don’t make the local paper, but they leave lasting impressions on those who see the fallout firsthand.
In the end, careful storage and good habits build a culture of responsibility. It’s about practical steps—dry rooms, intact labels, gloves on hands—not about memorizing arcane guidelines. Newcomers and seasoned chemists alike keep each other honest. The risk drops, and everyone goes home safe at the end of the shift. That, in my book, matters most.
Most chemical names slip right past us, but 2-Propylimidazole stands out to folks who spend time around laboratories or manufacturing. Staring at this chemical's MSDS sheet got me thinking—hazard symbols grab your attention for a reason. Everyday work with 2-Propylimidazole means thinking about more than just ticking off some regulatory box. This compound carries dangers you can’t just ignore, and ignoring safety gets people hurt.
Direct skin contact may burn or irritate. I once watched a coworker brush off a chemical splash, only to feel a rash develop by lunch. 2-Propylimidazole will do the same if you handle it barehanded, all in the blink of an eye. Your skin serves as your main barrier. Overlook gloves, and that barrier springs a leak. Breathing in dust feels a little less serious at first, but anyone who's had a cough from chemical exposure knows how fast things can go south.
No one expects an accident. You show up for your shift and figure you know the ropes—till the wrong move lands you in the ER. 2-Propylimidazole isn’t some mystery powder you can treat like flour. Its vapor or dust can bother your throat or nose before you even realize something is wrong. That discomfort turns into something more lasting if repeated exposures pile up. Workers have ended up with chronic skin conditions or breathing trouble because shop routines forgot the basics.
Chemicals like this find their way into production lines, specialty labs, or even research benches. Some companies make the mistake of quick handoffs and loose storage practices—one poorly labeled container, and a new worker gets a nasty surprise. That’s not an abstract risk. In my time around labs, I’ve seen labels fall off and folks mix up containers. If this compound ends up anywhere but a tight, dedicated cabinet, the risk grows every minute.
Putting on gloves and goggles isn’t about paranoia. Folks use these safeguards because they work. Chemical-resistant gloves create a line of defense for skin, and splash goggles block stray drops before they speed toward your eyes. Nitrile gloves hold up well under typical conditions with 2-Propylimidazole—stay away from plain latex, which won’t last. I always opt for a lab coat or apron, knowing no shirt keeps out a splash.
Inhaling powder is less likely with decent ventilation. Good airflow keeps contaminant levels down. If fans fail or a chemical hood gets blocked, exposure jumps fast. I learned early on to check airflow with a tissue—if it’s not being pulled in, step back and fix it. Respirators aren’t for show. In areas where dust builds, wear a mask rated for organic vapors and particulates.
Storage changes the whole game. Containers must seal tightly, with clear labels telling anyone what’s inside. I always return spare bottles to a dedicated cabinet, away from acids or anything you wouldn’t want mixing. Emergency showers, clean water for skin and eyes, and knowing where first aid sits keep a simple mistake from turning tragic.
2-Propylimidazole isn’t rare in chemical processing, but treating it as “just another powder” leads to problems. People who’ve seen real accidents don’t cut corners—each warning on the safety sheet comes from facts, not caution gone wild. By forming solid habits—washing up, checking PPE, labeling everything, and listening to that tickle in your throat—you lower risk for everyone around you. Chemicals reveal the need for respect every day, and staying safe never goes out of style.
Chasing a pure chemical—like 2-Propylimidazole—reminds me of cooking from scratch. It’s the difference between a home-cooked meal and fast food. For labs and industries, purity isn’t a buzzword. 2-Propylimidazole usually comes with purity above 98%, many times hitting 99% or better. I’ve seen how that extra percentage point can make or break a synthesis in organic chemistry. Even a tiny impurity can throw off experimental results, ruin a batch of specialty polymers, or quietly sabotage a pharmaceutical route that costs thousands in time and materials.
Specification sheets paint a careful picture: appearance (usually off-white to pale yellow solid or powder), melting point (often in the 110-115°C range), and strict limits on heavy metals and moisture content. It might look boring to the outsider, but chemists comb over those numbers. An odd melting point or a slightly darker shade may hint at trouble. Water content often gets measured down to fractions of a percent, since even modest humidity can mess with storage, formulation, or reaction yields.
Years back, I learned why packaging can’t be an afterthought. I’ve seen what happens to sensitive chemicals shipped in the wrong kind of bottle—clumping, color changes, sometimes even worse. 2-Propylimidazole doesn’t fare well when exposed to moisture, so it’s often packed in tightly sealed HDPE or glass bottles. Cap liners matter more than anyone admits. Vendors ship it in quantities from 25 grams up to 25-kilogram fiber drums, but it isn’t just about the size. It’s about keeping air and water out. Even trace moisture can creep in through a cheap plastic bag, so quality producers go for double-sealed bags or vacuum packaging.
Labeling tells the saddle story: lot number, production date, and purity prominently displayed. The hazard symbols also stare you in the face to remind handlers that safety comes first. For global shipping, secondary containment—like UN-certified drums or extra-strong outer cartons—prevents leaks or cross-contamination. Cutting corners here leads to chaos. I've seen lost money and wasted time from failing seals and broken bottles that left a sticky, hard-to-clean mess.
Purity and packaging flow into each other. If someone orders 99% pure material but it comes sweaty with condensation, confidence in any experiment goes down the drain. Labs and manufacturing setups rely on traceability—batch numbers, tight packaging, and reliable purity data. One botched bottle can set an entire team back by days. Modern quality management depends on keeping records straight and product consistent, not just rushing bottles out the door.
Stronger standards offer the only real fix. It helps when suppliers back up their numbers with certificates of analysis that include not just purity but details on possible trace contaminants. Some labs now run their own spot tests right as they unbox a shipment, just to be sure. Maybe we’ll see more tamper-evident seals and real-time temperature or moisture data tags on containers, since every improvement cuts out one more source of doubt.
Supply chain hiccups have shown how easy it is to lose track of quality between source and destination. Better packaging and clear transparency give customers the tools for real chemistry instead of guesswork. No one likes a surprise in science, especially not from their main ingredient.
| Names | |
| Preferred IUPAC name | 2-propyl-1H-imidazole |
| Other names |
2-Propylimidazole 2-Propyl-1H-imidazole |
| Pronunciation | /tuː-ˈproʊ.pɪl.ɪˈmɪ.dəˌzɒl/ |
| Identifiers | |
| CAS Number | 2887-89-0 |
| 3D model (JSmol) | `3DModel:JSmol/CCCN1=CN=CN1` |
| Beilstein Reference | 87368 |
| ChEBI | CHEBI:85155 |
| ChEMBL | CHEMBL132206 |
| ChemSpider | 77327 |
| DrugBank | DB08303 |
| ECHA InfoCard | 21e2cfae-5ada-4e43-ab7d-8a6896bbf13f |
| EC Number | 22318-94-7 |
| Gmelin Reference | 89231 |
| KEGG | C18503 |
| MeSH | D016693 |
| PubChem CID | 120425 |
| RTECS number | UY4375000 |
| UNII | 62Q8R6F79W |
| UN number | UN3276 |
| Properties | |
| Chemical formula | C6H10N2 |
| Molar mass | 110.16 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | amine-like |
| Density | Density: 1.01 g/mL at 25 °C (lit.) |
| Solubility in water | Soluble |
| log P | 0.27 |
| Vapor pressure | 0.0025 mmHg (25°C) |
| Acidity (pKa) | 14.5 |
| Basicity (pKb) | 7.99 |
| Magnetic susceptibility (χ) | -5.7 × 10^-6 cm³/mol |
| Refractive index (nD) | 1.485 |
| Viscosity | 1.07 mPa·s (25°C) |
| Dipole moment | 2.97 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 185.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -19.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4577.7 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Danger |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P310, P362+P364 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 107°C |
| Autoignition temperature | 430 °C |
| Explosive limits | Explosive limits: 2–12% |
| Lethal dose or concentration | LD50 oral rat 650 mg/kg |
| LD50 (median dose) | LD50 (median dose) for 2-Propylimidazole: "566 mg/kg (oral, rat) |
| NIOSH | NIOSH QV0400000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 mg/m³ |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Imidazole 1-Methylimidazole 2-Ethylimidazole 2-Butylimidazole 2-Phenylimidazole |