Decades ago, chemists digging into the subtle ingredients that make foods taste the way they do stumbled onto 2-Propyl pyrazine. It cropped up during explorations into roasted flavors, coffee, and chocolate aromas. As chemical analysis tools got sharper through the late 20th century, researchers started seeing that pyrazines represented a broad group of molecules appearing wherever heat and natural sugars mixed. 2-Propyl pyrazine came into focus as both industry and academia dug deeper into flavor science, always hunting for the molecular roots of “nutty” and “roasted” notes. By the 1980s, flavor houses picked up this compound for the toolbox. Since then, interest has only grown, driven by the food industry’s unending appetite for reliable, natural-feeling additives that pass muster with shifting regulations and consumer worries.
Anyone who’s worked in a flavor lab or walked through the back rooms of a coffee roaster has probably caught a whiff of 2-Propyl pyrazine. This chemical doesn’t jump out at you in a pure form — the concentration in foods is usually tiny — but dial it up, and a signature nutty, baked aroma creeps into the air. Food technologists reach for this compound when replicating flavors that recall toasted seeds, browned grains, peanut brittle, or even the edge of caramelized onions. Beyond food, research labs have poked at its utility in environmental monitoring and chemical trace analysis, though food tech dwarfs these other uses by a country mile.
2-Propyl pyrazine is a colorless to pale yellow liquid, carrying a boiling point of roughly 181°C and a molecular weight just shy of 150. Tucked away behind the simple formula (C7H10N2), the structure features a six-membered pyrazine ring, with a snappy little propyl tail at the second carbon. Its vapor pressure sits high enough to make effective aroma delivery in food systems a breeze but low enough to prevent it from flashing off in open air. Solubility can trip up anyone trying to blend it with aqueous products, since 2-Propyl pyrazine prefers organic solvents over water. For technicians, the volatility and odor threshold offer a delicate balancing act: too little, and it stays buried; one drop too many, and it overwhelms everything in its path.
Anyone ordering it in bulk spots the CAS number 13925-00-3 on the drum, along with purity figures often topping 98%. Labs demand proper chromatographic identification — usually GC/MS — to verify the compound and rule out any shadowy byproducts or alkyl-substituted cousins. For regulatory purposes, flavor-grade 2-Propyl pyrazine arrives with Food Chemicals Codex credentials, batch test certificates for residual solvents, and allergen declarations that keep things above board. Labels in the US or EU often list it as “Flavoring Substance” or by its specific name, ensuring compliance with lists like FEMA GRAS or the Regulation (EC) No 1334/2008. Each label reveals a trail of paperwork, making ingredients traceable and audits a little less painful.
The classic lab synthesis begins with pyrazine as a scaffold and slaps on a propyl group using straightforward alkylation. Most industrial outfits lean on Friedel-Crafts alkylation routes or catalytic hydrogenation, using propyl halides as starting material and leveraging common acid or base catalysts. The process runs at moderate temperatures, designed to minimize side reactions, since purity proves crucial in flavor work. Chemical manufacturers sometimes tweak the route slightly to dodge problematic intermediates or increase yield, but at its core, the process draws on reliable organic chemistry, easily scalable from beaker to reactor.
Chemists don’t leave well enough alone, so they’ve tinkered with 2-Propyl pyrazine to craft longer or branched alkyl chains, hoping to widen the library of aroma nuances. Substituting at other positions on the pyrazine ring doesn’t just change the smell and taste — it can shift the volatility, making compounds more or less useful in different applications. The core pyrazine ring resists heavy modification, staying pretty robust in the face of standard heat, light, and mild acids or bases. That durability translates into shelf stability, a key requirement for anything headed into mass-market food manufacturing. Labs occasionally use 2-Propyl pyrazine as a precursor for syntheses chasing down more complex heterocyclic compounds, but the beaten path keeps leading back to flavor engineering.
This molecule wears a few hats. In catalogs and safety reports, you’ll spot it as 2-n-Propylpyrazine, 1-Pyrazine, 2-propyl-, or just “pyrazine, 2-propyl-.” Trade names from specialty suppliers sometimes riff on these to sound unique or proprietary, yet the core identifiers stay the same to keep customs agents and warehouse teams on the same page. FEMA 3182 marks its main flavor industry registration, and you’ll occasionally find local names in regional markets, though most distributors stick to the IUPAC or direct synonym forms.
As is often the case with strong-smelling substances, a little goes a long way, and that holds true for human health too. 2-Propyl pyrazine rarely causes problems at flavor-level doses, but lab workers and blend operators stick to gloves, goggles, and fume hoods, since skin contact can provoke mild irritation and the vapor can build up in closed spaces. Current GHS hazard statements peg it as a low-toxicity compound, but the rules for safe handling match the norms for aromatic chemicals: proper ventilation, PPE, and spill response protocols. In regulated food supply chains, routine batch testing screens for known contaminants, and cross-contact with undeclared allergens is prevented by batch segregation. Training plays a role, with everyone from junior techs to QA officers familiar with safe storage and transportation.
Walk into factories making roasted nuts, baked snacks, processed meats, or even pet food, and chances are good you'll spot a jug or drum of 2-Propyl pyrazine somewhere along a production line. Food technologists lean on it to re-create natural roasting profiles, bump up browned notes in shelf-stable products, or round out artificial peanut butter. Some beverage formulations, especially those fiddling with malt or coffee flavors, turn to this molecule for depth. Beyond food, analytical chemists use 2-Propyl pyrazine as a trace marker for quality control or research into Maillard reaction byproducts. A few agricultural and environmental studies look at its presence in soils or plant emissions, but the numbers remain small compared to the flavor industry.
Researchers continue picking apart how 2-Propyl pyrazine forms during real-world cooking and roasting, hoping to mimic these mechanisms in controlled industrial processes. Academic groups chase after the pathways that produce it during Maillard and caramelization reactions, testing varied sugars, amino acids, and process tweaks to crank up yields or steer flavor outcomes. Innovation on the production side focuses on greener synthesis — reducing the use of halogenated solvents or tracking down routes that start from bio-based precursors. In the background, a constant current of regulatory and safety assessment flows in the literature. Scientists correlate minute differences in concentration with shifts in perceived flavor, while sensory panels, advanced chromatography, and even machine learning enter the picture for more precise formulation.
Anyone in food safety or toxicology circles keeps a close eye on whether high doses of aromatic chemicals like 2-Propyl pyrazine pose risks over time. Existing studies, many sponsored by large food companies or independent research groups, point out that acute toxicity sits low; animals tolerate quantities hundreds of times higher than any daily intake. Reviews cover short- and long-term ingestion, looking for signs of liver, kidney, or reproductive impacts. Most government panels arrange regular re-assessments to catch up with emerging data — including rare allergy reports or unexpected contaminants — but so far the green light for flavor use remains steady. Animal testing still forms much of the backbone here, though computational models and in-vitro screening slowly take on a bigger share, especially as calls for animal-free research grow louder. Caution surfaces in every technical meeting, since regulatory limits may change with every new review.
Industry voices in taste and scent innovation urge a steady search for new uses of 2-Propyl pyrazine, especially in plant-based product development, where bridging the gap between “meaty” and “veggie” sits as a stubborn flavor challenge. As food consumers zero in on “natural” labels, pressure grows to wring 2-Propyl pyrazine out of sustainable, traceable production, with fermentation and bio-catalysis held up as promising tools on the horizon. Supply chains may eventually lean on digital twin monitoring or AI-powered synthesis routes to knock out steps that generate waste or touch on regulatory red flags. Meanwhile, researchers in environmental chemistry keep taps on how pyrazine compounds show up as trace contaminants, nudging regulatory frameworks to keep pace with discoveries. Future development hangs on balancing what works in the lab, what regulators allow, and what consumers trust. As the food, chemical, and analytical worlds draw closer together, the role of this humble molecule will keep evolving, rolling along as an unassuming but key part of modern flavor chemistry.
Most folks don’t talk about 2-Propyl Pyrazine at the dinner table, but the molecule sneaks into plenty of our food memories. Scientists call it a flavor compound. To everyday eaters, it’s the spark behind the nutty, roasted aroma in a cup of coffee or the mysterious toasty note in a chocolate bar. This chemical, found in trace amounts in foods that have been roasted, baked, or grilled, shapes how we experience flavor, even if it never lands on a menu.
I grew up in a house where my father roasted peanuts every Sunday. The smell lingered all afternoon. After some digging in food science journals, I found that chemists praise 2-Propyl Pyrazine for its intense, pleasant scent. Labs can synthesize it with precision, which helps the food industry maintain consistency from batch to batch. It gets included in everything from savory snacks to baked treats. Cereal brands, for example, rely on it to pull off a convincing roasted grain flavor, even if they use more processed or bland base ingredients.
Food isn’t the only place this compound shows up. You’ll find traces in cigarette smoke and even in some pharmaceuticals meant to mask bitterness. The reason is simple—humans connect roasted and nutty notes to comfort and satisfaction. A few molecules of this compound can tip a coffee extract from dull to crave-worthy, or take a fake chicken nugget just a step closer to real.
Not all raw materials taste the same every harvest. One year, you have cocoa beans that miss their usual aroma, or peanuts that just taste flat. Food makers can’t gamble on nature’s whim, especially with global recipes and expectant customers. 2-Propyl Pyrazine covers for those off-years. It makes it possible to lower costs—improving flavor with smaller amounts of expensive natural ingredients. For folks on plant-based diets, it steers alternative proteins toward something familiar.
The use of synthesized flavors also keeps large food producers out of trouble with spoilage or seasonal shortages. In my own experience working in a bakery, we struggled with inconsistent nut flavors after a bad harvest. Supplementing with this compound let us keep our recipes in line, so regulars never noticed the difference.
Dependence on artificial flavors raises questions. Long term exposure is still an open field for scientists, although agencies like the FDA currently label 2-Propyl Pyrazine as safe in the amounts used. More than once at food trade shows, I’ve heard chefs and enthusiasts ask for clearer food labeling. They want to know what’s real and what’s been added for effect.
Pushing for transparency helps consumers make choices that reflect their values. Besides, talent in the kitchen deserves a spotlight. Real roasting produces depth that can’t be bottled. On the other hand, synthetic flavors help close gaps in supply and cost. Maybe the sweet spot sits where companies balance honest labeling, boost natural flavors through good farming and roasting, and call on compounds like this sparingly. Sharing the story behind each bite—from the science lab to the home kitchen—invites everyone to see flavor as both craft and chemistry.
2-Propyl pyrazine isn’t a household name, but anyone who dabbles in flavor chemistry or fragrance research gets familiar with compounds like this. A little background: it’s part of the pyrazine family—chemicals with a strong influence on taste and scent, sometimes sweet, sometimes earthy. The story of 2-propyl pyrazine often starts at the molecular level, and attention always turns to its formula: C7H10N2.
That formula, C7H10N2, says a lot. There are seven carbons, ten hydrogens, and two nitrogens in every molecule. The ring structure gives it a unique shape, which means a unique set of properties. Those in the food industry, perfumery, and even pharmaceuticals chase after this kind of information. It’s the difference between a flavor note that makes a dish memorable, or a scent that powers up a designer perfume.
Molecular weight is the next piece of the puzzle. For 2-propyl pyrazine, it sits at 122.17 grams per mole. Calculating this isn't just a math drill from high school. These numbers change how chemists think about reactions, purity, how a substance travels, and even regulations. I still remember my first run-in with a mis-labeled bottle in an undergraduate lab. A wrong molecular weight led to a chain of messy calculations and a week’s worth of re-doing work. Details matter—especially for researchers measuring out tiny, nearly invisible quantities.
Outside schools and labs, 2-propyl pyrazine pops up behind the scenes. It helps shape the flavors of roasted coffee, baked goods, and some chocolates. Manufacturers rely on the exact formula and molecular weight to decide how much to add, how to test for safety, and what kind of flavor profile gets delivered to consumers. There’s no room for guesswork in a food plant where one slip means a product recall.
Looking at regulations, chemical identity stands as the first step. Food safety authorities want everything clear: what is in the product, how much, and whether it’s safe. Miss out on the exact formula, and producers tangle with compliance issues. Some companies have learned it the hard way, pulling batches after tests uncovered inconsistencies with the chemical makeup they declared.
An issue I’ve seen involves sourcing. As demand for natural or nature-identical flavors grows, companies often go global. Sourcing channels stretch from country to country, turning consistency into a challenge. Reliable reference materials, tested and published chemical data, and clear labeling on all shipments help everyone keep standards high.
Education comes next. Every new chemist, flavorist, or regulatory specialist benefits from a deeper handshake with chemical formulas and molecular weights. Workshops, digital resources, even basic lab experience pay off. I’ve seen junior team members come up with creative fixes because they understood the underlying chemistry, not just the recipe card.
For anyone looking to build safer, tastier, or more sustainable products, accuracy on molecular formulas and weights stands as a foundation, not trivia. Teams need solid, well-researched data, routine verification, and a genuine interest in the real stuff behind a label. It’s the only way to keep quality high and surprises low.
2-Propyl pyrazine shows up on the ingredient lists of many products with roasted, nutty, or earthy notes. Walk into a bakery or a coffee shop and those subtle, toasted undertones in your favorite treat might owe something to this compound. Manufacturers use it to enhance or mimic flavors that would otherwise require lengthy roasting or to bring depth to processed foods where fresh ingredients alone can’t pull it off.
Whenever I spot a chemical-sounding name on my food label, I get curious. Turns out, food safety agencies around the world keep a close watch on what goes into our food, especially with flavoring agents like 2-propyl pyrazine. In both Europe and North America, food safety bodies such as the US Food and Drug Administration (FDA) and Europe’s EFSA have classified it as a permitted flavoring substance in limited amounts. They didn’t find it mutagenic, carcinogenic, or likely to accumulate in dangerous amounts if used within set limits. Animal tests didn’t show worrying effects at the levels used in food, and the compound doesn’t hang around in the body for long.
People get nervous at the sound of a chemical name, but it helps to remember that “natural” and “synthetic” aren’t always markers of safety. 2-Propyl pyrazine also exists naturally in roasted foods and even coffee. Regulators looked at how much people consume by eating a typical mix of snacks, cereals, and roasted foods. Average daily intake is nowhere near the level that triggers adverse effects in studies.
In practice, 2-propyl pyrazine lets food makers create reliable flavors that would otherwise be harder to control or expensive to produce from raw materials. This consistency supports both large and small-scale producers who want to deliver familiar taste experiences. As someone who enjoys trying foods from both supermarket shelves and local shops, I’ve noticed that flavor enhancers help level the playing field between big brands and startups.
Food technology evolves, but the memory of a favorite snack’s taste stays constant partly thanks to molecules like this one. Flavor isn’t just about pleasure—it shapes our identity and family traditions. There’s value in being able to recreate comforting tastes from childhood, especially for communities trying to maintain culinary traditions away from home.
Some folks see a long chemical name and can’t help but worry. I’ve seen heated social media debates driven by little more than a scary-sounding term on the back of a chip bag. Here’s where better labeling can make a difference. More producers now share plain-language explanations, making it easier to understand why something is there. Trust grows when people see a clear reason for every additive and know it passed safety checks.
The world doesn’t stand still, and neither does ingredient science. New research keeps checking flavor compounds for risks that earlier studies might have missed. Ongoing monitoring helps catch any long-term issues early. If fresh evidence emerges, regulations adapt. For now, transparency and quality control act as the strongest shields for the public. I look for producers who go beyond minimum standards, testing raw materials and training staff to avoid contamination. Honesty on labels and open explanations lead to smart choices at the grocery store.
2-Propyl Pyrazine isn’t some household pantry staple. Walk into a lab or a flavor manufacturing plant, and you might find a container on a shelf, tucked away from the busy main aisles. People know it for the roasted, nutty notes it brings to foods, perfumes, and chemical experiments—but that punchy aroma comes with its own side of responsibility.
Over my years wrestling with chemical storerooms, I’ve seen corners cut. Someone leaves a cap loose or stashes a small bottle wherever it’ll fit. Those short-cuts tend to invite problems. 2-Propyl Pyrazine prefers a cool, dry place—think ziplocked away from direct sunlight, not tossed beside cleaning sprays or open drains. A lot of chemical bottles share a “keep out of direct sunlight” warning, but here it's more than advice. Heat tends to change a chemical’s temperament; in this case, it can mean stronger fumes, ruined batches, or worse.
Not every shelf works either. I reached up once and watched a glass stopper tumble after someone put a bottle too close to the edge. Any volatile compound, including 2-Propyl Pyrazine, should stay well back from the ledge. Sealed containers built with chemical resistance stand guard against leaks or accidental knocks. Metal shelves offer a sturdy base, but I trust my favorite plastic bin—dedicated for these types of aromatics—because it shrugs off spills without rusting and wipes clean fast.
Anyone who’s worked with chemicals knows the temptation to skip gloves on a quick task. Familiarity breeds carelessness, yet 2-Propyl Pyrazine doesn’t care if it’s your fifth or fiftieth time pouring. Even tiny spills leave a lingering, earthy tang in the air, and skin contact means days of irritation. Nitrile gloves and safety glasses stay non-negotiable in my book. A fume hood adds another layer, capturing those invisible vapors so they don’t wander into open workspace or recirculating air systems.
I once shared a workspace with folks who didn’t believe in proper labeling. That naive confidence leaves everyone guessing. Clear labels, including the date received and opened, help track freshness and safety—no one likes a surprise from a bottle that isn’t what it seems. I always store 2-Propyl Pyrazine away from strong acids, oxidizers, or bases. Even one accidental mix can trigger a dangerous reaction. Dedicated, separated shelves give me some peace of mind, especially in crowded storage areas where bottles rub shoulders all day.
No storage or handling routine feels complete without a cleanup plan. Even the most practiced hands drop things. I learned early not to trust loose paper towels with chemical spills. Spill kits built for organic liquids stay within arm’s reach—absorbent pads, chemical-rated trash bags, and a clear process for disposal. After cleaning, every tool and glove heads straight into a waste container, not the regular trash bin where they might pose risk to someone else.
Ventilation remains the silent hero. I once worked in a cramped basement lab with a single fan. Endless headaches—not from overwork, but from building fumes. Decent airflow clears out the fog and makes differences in day-to-day health and alertness. You want a workspace that smells like air, not burnt popcorn or nuts.
2-Propyl Pyrazine shows up in everything from snacks to experiments because of its powerful scent, but its handling shouldn’t be casual. Regular safety drills, refresher courses on personal protective equipment, and a healthy respect for chemical boundaries keep everyone safer. Those little routines—seems boring at first—end up saving costs, time, and sometimes lives down the road.
Take a whiff of roasted peanuts or a bowl of grilled corn, and you’re already in the territory of 2-Propyl Pyrazine. If you haven’t worked with the stuff, maybe you’ve at least eaten a snack where it played a supporting role. It’s that unmistakable blend of roasted, nutty, and almost earthy notes. Some folks say it’s like a walk through a bakery just after a batch of dark bread comes out of the oven. Others catch a hint of grain silo, that dry and slightly musty but oddly inviting note in the background.
I first picked up the aroma of 2-Propyl Pyrazine in a product development lab. We were chasing after the sensory profile of oven-roasted chickpeas for a snack project. Just a tiny drop on a scent blotter filled the room with a strong, nutty smell that had the sharp edges of roasted coffee beans. I remember how quickly it overpowered milder smells. My colleagues could spot it instantly in a lineup. This kind of sensory impact helps a lot when replicating food aromas—the right note of authenticity makes snacks pop.
2-Propyl Pyrazine belongs to a family of molecules responsible for the “Maillard reaction” aromas. Anytime a cook browns food, pyrazines show up. Studies on pyrazines reveal their pivotal part in everything from chocolate to roasted meats. The strong nutty and toasted character of 2-Propyl Pyrazine comes paired with a subtle undertone of earthiness. Sometimes people even call it woody, bordering on a damp forest floor in fall. Not many food flavor molecules deliver the same roasted punch without dragging along off-notes like burnt rubber or excessive bitterness. Formulators rely on it to bring life to plant-based meats or to boost less expensive coffee blends.
Flavor creators hit roadblocks if they use too much 2-Propyl Pyrazine. Open a bottle, and the whole room starts smelling like burnt toast within minutes. Overdose it, and the note can go from inviting to overwhelming. This has happened to me—during taste panels, a batch of flavored nuts went from “roasted fresh” to “campfire accident.” Balance is hard, especially since people sense this molecule at tiny levels.
Quality and sourcing also matter. Poorly handled pyrazines start to smell stale or chemical. I’ve dealt with low-quality supply chain samples that introduce strange metallic notes no roasted peanut ever needed. Sourcing high-quality aroma chemicals makes a difference, as does storage in well-sealed containers away from heat and light.
Education in the food industry still lags behind. I’ve watched food technologists discover the power of 2-Propyl Pyrazine to upgrade everything from plant-based snacks to energy bars. More precise dosing solves most of the common complaints. Sharing sensory standards in team training sessions gives everyone a real sense for where the right threshold sits.
Taste and recall studies show most consumers link pleasant roasted and nutty smells to comfort foods. Harnessing this molecule thoughtfully can trigger nostalgia and trust—a big edge for brands looking to tap into flavors that feel familiar and satisfying.
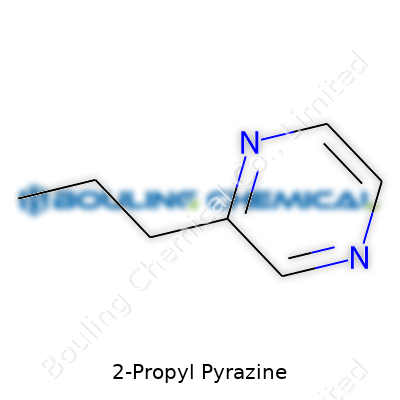
| Names | |
| Preferred IUPAC name | 3-Propylpyrazine |
| Other names |
2-n-Propylpyrazine Pyrazine, 2-propyl- 2-Propyl-1,4-diazine |
| Pronunciation | /ˈtuː ˈproʊ.pɪl paɪˈreɪˌziːn/ |
| Identifiers | |
| CAS Number | 18138-04-0 |
| Beilstein Reference | 1718731 |
| ChEBI | CHEBI:89776 |
| ChEMBL | CHEMBL137657 |
| ChemSpider | 182434 |
| DrugBank | DB14659 |
| ECHA InfoCard | ECHA InfoCard: 100.116.942 |
| EC Number | 219-934-6 |
| Gmelin Reference | 109814 |
| KEGG | C18697 |
| MeSH | D017929 |
| PubChem CID | 142972 |
| RTECS number | UB6250000 |
| UNII | 6Z73CU78K9 |
| UN number | UN3295 |
| Properties | |
| Chemical formula | C7H10N2 |
| Molar mass | 150.22 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | nutty, roasted, popcorn |
| Density | 0.967 g/mL at 25 °C |
| Solubility in water | Slightly soluble |
| log P | 1.44 |
| Vapor pressure | 0.0262 mmHg at 25 °C |
| Acidity (pKa) | 15.0 |
| Basicity (pKb) | 2.39 |
| Magnetic susceptibility (χ) | -68.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4870 |
| Viscosity | 1.010 mPa·s (25 °C) |
| Dipole moment | 1.86 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 222.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2.47 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4116 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | C1=CN=CC(NCC)=N1 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: "P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0-🛢 |
| Flash point | 76 °C |
| Autoignition temperature | 400°C |
| Explosive limits | Explosive limits: 1.6% - 11.9% |
| Lethal dose or concentration | LD₅₀ (oral, rat): 460 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 2200 mg/kg |
| NIOSH | NT8050000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100 |
| Related compounds | |
| Related compounds |
2-Ethylpyrazine 2-Methylpyrazine 2-Isopropylpyrazine 2-Sec-butylpyrazine |