The story of 2-Piperidinoethanol catches my attention not because it's the most famous molecule in organic chemistry, but because its path weaves together the history of medicinal chemistry and industrial synthesis. Chemists first noticed this compound in the early boom days of heterocyclic research. After World War II, laboratories racing to innovate with nitrogen-based rings spotted the value of combining piperidine with short alcohol chains. This period, rich with experimentation, led to both obscure and blockbuster drugs, but also set new standards for purity and safe handling. The attention 2-Piperidinoethanol earned in those days came from its potential as a building block for both pharmaceuticals and intermediates in complex syntheses, as chemists pushed for increasingly powerful and selective agents. It’s the type of compound that doesn’t get paraded in magazines, yet it quietly helped researchers move from broad-stroke experimentation to more targeted drug design.
The average person doesn’t spot 2-Piperidinoethanol by name on the drugstore shelf. In research and manufacturing circles, the organic backbone of piperidine rings with an ethanol side chain shows up in technical documents, supply catalogs, and internal process flowcharts. Most chemists recognize it as a clear, colorless, and sometimes slightly viscous liquid—its presence a sign that the lab operates with a deep portfolio of synthetic strategies. Chemical suppliers offer it at purities suitable for both bench chemistry and scale-up, where consistency really matters. This compound finds a home in pharmaceutical, agrochemical, and fine chemical research, not because of flash, but because it helps connect the dots between basic building blocks and final products that drive real-world innovation.
Anyone who’s had to pour out 2-Piperidinoethanol from a bottle immediately notices its mild amine odor and the way it blends with water. Boiling at around 237°C, this liquid displays decent stability under ordinary laboratory conditions. I see value in this midpoint between volatility and viscosity—it neither evaporates away at room temperature nor sits unmoving like heavy oils. Its molecular formula, C7H15NO, reflects a simplicity that belies its reactivity: one can spot both the nucleophilic nitrogen and the flexible hydroxyl on its ethanol tail. As for density, it hovers close to 0.97 g/cm³, and the refractive index lands near 1.469, which hints at its moderate interactions with both polar and nonpolar solvents. Compared to many secondary amines, its water solubility deserves a mention, opening up easier handling for process chemists.
Reliable labeling for 2-Piperidinoethanol forms the backbone of both safe handling and regulatory compliance. Bottles come marked with batch number, purity level, and identifiers like CAS number 3203-38-9. Purity for research or industrial use usually reaches 98% or higher, and suppliers often provide gas chromatography or HPLC certificates. Labels flag corrosiveness with hazard pictograms, alerting users to the risks posed by both basic amines and the irritation potential from alcohols. Safety Data Sheets arrive with the shipment by law, detailing handling, storage, transport, and disposal requirements. Over the years, I’ve learned to trust companies who document not just the source, but also stipulate the methods used in testing for heavy metals, moisture, and potential byproducts. These details matter when translating bench results to production-scale operations.
Talking to old-timers in the industry, it’s clear that methods of preparing 2-Piperidinoethanol have come a long way. Early routes involved the slow and sometimes messy reaction of piperidine with ethylene oxide. This classic nucleophilic attack gives the C-N bond that’s at the heart of the molecule. Over time, chemists fine-tuned reaction temperatures, solvent choices, and purification steps to crank up yields and minimize byproducts. Catalysts and process controls helped lower energy demand and improve safety—a big leap for production facilities. While many textbooks still mention batchwise synthesis, continuous-flow processes now help larger players keep up consistency on a tonnage scale. Environmental compliance pressures pushed improvements, too. Waste minimization and solvent recycling now get baked into process design, reducing the ecological footprint of every kilogram produced.
If you ask anyone involved in custom synthesis, 2-Piperidinoethanol keeps showing up as a flexible intermediate. Its structure essentially holds two functional groups destined for modification—the secondary amine and the primary alcohol. Each can lead down a separate path for further reaction. Acylation at nitrogen produces a wide variety of amides used in medicinal chemistry, especially in CNS-active drugs. Quaternization turns the nitrogen into a permanently charged center, opening routes into surfactants and cleaning agents. On the other end, the alcohol group offers another handle. Oxidation leads to the corresponding aldehyde or acid, cyclization produces heterocyclic derivatives, and etherification extends the molecule for new properties. The chemistry here allows both fine-tuning and drastic changes, so the compound’s popularity in chemical R&D is easy to understand.
A single name doesn’t always cover the full range of references for 2-Piperidinoethanol. Synonyms in literature and product catalogs include 1-(2-Hydroxyethyl)piperidine, N-(2-Hydroxyethyl)piperidine, and 2-(Piperidin-1-yl)ethanol. Depending on the supplier, shorthand abbreviations or catalog codes occasionally tag along, just as chemists sometimes scribble “PEE” or “HEPIP” in notebooks to save space. For regulatory work or international shipping, the CAS number serves as the common language, letting experts sidestep confusion from translation differences or local product names. This diversity in naming speaks to the history of discovery and the spread into global commerce.
Safety rules for handling 2-Piperidinoethanol don’t feel optional to anyone who’s dealt with basic amines. Gloves and goggles become part of routine lab work, given the compound’s potential to irritate eyes, skin, and mucous membranes. Spills on benches produce not just strong smells but actual hazards—both corrosive action and volatile amine vapors. Ventilation matters in both labs and factories, where even modest concentrations could pose respiratory risks. Storage calls for tightly sealed bottles away from strong acids and oxidizers, with labels warning about possible environmental harm from improper disposal. Over the years, global standards evolved, reflected in requirements set by agencies like OSHA and ECHA, but local enforcement and company-specific guidelines ultimately drive everyday behavior in the workplace. This tight regulation stems from hard lessons learned in the past, where accidents and exposures shaped today’s cautious approach.
My work has exposed me to many contexts where 2-Piperidinoethanol plays a quiet but meaningful role. In drug development, it rarely ends up in the pill itself, but rather helps synthesize precursors, especially for antihistamines and CNS-active compounds. Its ability to bridge alcohol and amine reactivity makes it a frequent stop on complex multistep syntheses. Looking farther, it acts as a catalyst precursor and stabilizer in polymer chemistry. Some specialty coatings and resins count on its flexibility to introduce piperidine rings into tighter crosslinked networks, boosting performance in demanding environments. In agricultural chemistry, modifications lead to new pesticide candidates. Research circles experiment with it in chromatography as a mobile-phase additive, thanks to its basicity and solubility. No single blockbuster use dwarfs the others—a sign of a compound that finds its place by quietly enabling better science instead of seeking a headline.
R&D groups spend time probing the limits of 2-Piperidinoethanol in both new syntheses and material science. Universities and contract labs seek derivatives that work as active pharmaceutical intermediates, while industrial teams look for improvements in synthesis efficiency, yield, and environmental impact. Researchers continuously develop more selective catalysts for functionalization, aiming for both greener chemistry and reduced cost. Patent filings sometimes hint at new push-and-pull between the amine and alcohol groups to bridge complex molecular structures. The search for more bioavailable or more stable derivatives often relies on taking this relatively simple molecule and pushing its boundaries. In my circle, I see chemists evaluating not just reactivity, but also solubility, toxicity, and compatibility with automated synthesis. Each breakthrough in this space doesn’t just improve 2-Piperidinoethanol’s profile—it opens doors for related families of drugs, materials, and specialty chemicals.
Toxicological studies of 2-Piperidinoethanol don’t suggest the same hazards as heavy metals or persistent solvents, but that doesn’t mean the compound gets a free pass. Acute exposure leads to irritation of skin and mucous membranes, and ingestion or inhalation could trigger nausea or central nervous system effects in higher doses. Chronic studies remain thin, revealing the need for more transparent publication of both workplace exposure data and animal studies. Regulatory bodies have set occupational exposure limits rooted in available data, but most recommendations err on the cautious side. Environmental fate draws interest as well, since amines sometimes persist or cause aquatic toxicity if released in volume. Labs and factories adopt a prevention mindset, focusing on containment and careful disposal rather than relying solely on post-exposure remediation. Advances in non-animal toxicity testing—an emerging topic—promise faster updates to workplace guidelines in the next few years.
2-Piperidinoethanol stands as an example of a compound whose future depends less on news headlines and more on the steady march of progress in chemical synthesis and regulation. As green chemistry takes on a larger role, pressure will build for cleaner, safer, and more energy-efficient methods to produce the compound at scale. The pharmaceutical industry may push for closer monitoring of impurities and innovative routes to higher-purity derivatives for new APIs. Specialty chemicals and advanced materials will continue driving molecular design in new directions, often using 2-Piperidinoethanol as a gateway to more complex piperidine-containing products. As regulation grows stricter and environmental standards tighten, both suppliers and users need to keep adapting. The lessons learned by generations of chemists—and the ongoing dialogue between research, industry, and regulators—shape the landscape. Staying ahead means investing in both technical expertise and responsible stewardship, building on what worked in the past but never resting on old habits.
Walk through a lab or flip through a pharmaceutical manual, you’ll run into a list of odd names. 2-Piperidinoethanol turns up more often than you might expect. This compound plays a supporting role in the backstage world of chemical synthesis. It’s not for everyday folks, but its impact shows up in places many would recognize.
Ask anyone working in a drug development lab, and they’ll say progress often depends on reliable building blocks. 2-Piperidinoethanol fits into this picture as a common intermediate. Organizations that produce antihistamines, antidepressants, or even local anesthetics have relied on this compound. It enters the scene for two main reasons: as a base structure in the molecule or as a helping hand for reaction steps.
Years back, I worked briefly in a small pharmaceutical startup. We tried to streamline a synthetic path for a new anti-inflammatory drug. Our chemist picked 2-Piperidinoethanol to tweak the side chain on a main scaffold. The flexibility this compound offers saved both time and money during that project. It taught me that seemingly simple chemicals play a role in making modern medicine affordable and available.
Pharmaceuticals grab headlines, but the uses don’t end there. 2-Piperidinoethanol also helps create chemicals that protect metal from rust, keep water systems clean, and make plastics tougher. Manufacturers count on this compound to act as a corrosion inhibitor additive in lubricants and coolants. It helps stop pumps and pipes from breaking down too soon. You want your equipment to outlast those warranty dates, and chemistry like this keeps things running.
Go over to the textile field, and its derivatives find their way into dyes. Coloring fabrics so shirts or pants keep their bright colors after washing really comes down to chemical stability, and ingredients like 2-Piperidinoethanol make this happen.
With any chemical, safety and environmental management matter. 2-Piperidinoethanol needs careful handling. Accidental skin or eye contact can cause irritation. Regulatory agencies such as the European Chemicals Agency have published guidelines on storing and using this compound. Companies track usage to meet both legal and ethical standards.
It also pays to think about waste. Factories using this chemical have started to recycle any leftover material instead of tossing it. The shift cuts down on environmental contamination and saves costs over time. Sharing best practices between industries and following established protocols reduces risks not just for workers, but for surrounding communities too.
The reach of 2-Piperidinoethanol spans research, production, and sometimes public health. Lately, research groups have been trying to develop greener pathways to make this compound. Some are exploring bio-based feedstocks and catalytic methods that use less energy. There’s a drive to push for innovation so that benefits don’t come at too steep an environmental price.
Chemicals like 2-Piperidinoethanol might not become household names, but the work done to improve their production has ripple effects. Every step toward safer methods and lower emissions means a little less stress on workers, air, and water. Keeping this conversation at the front helps make real progress in both health and industry.
2-Piperidinoethanol has a place in many labs, showing up in research as well as industry. Having spent time among flasks and bottles in a university chemistry building, I’ve learned not to trust any clear liquid simply because it doesn’t reek or look particularly dangerous. This one can irritate the skin, eyes, and even your lungs if you’re careless. Stories from co-workers remind me that working with chemicals like this always means something could go wrong in a hurry. There’s always someone somewhere who has underestimated the risks and paid the price.
Nobody strolls into a chem lab and splashes 2-Piperidinoethanol around with bare hands. Gloves make a huge difference. I’ve seen nitrile gloves handle the job for light contact, but thicker ones help for spills. Goggles shape daily lab fashion, whether mixing samples or rinsing beakers, because even a splash burns fast. If you ever saw a friend get a drop in the eye, you never skip this gear again. A lab coat closes that safety loop—my old coat bears scorch marks from long-ago projects, standing as proof that sleeves matter.
No one wants invisible vapors silently sneaking into their nose and mouth. Fume hoods offer protection. The hum of the extractor fan always reassures me that whatever I’m working with isn’t going straight into my lungs. On crowded days, folks line up for their turn, refusing to get lazy and shortcut workflow. Proper lab benches, clean surfaces, and secondary containers help everyone dodge accidental chaos. I always lay out extra absorbent pads under my glassware before opening anything even mildly risky to soak up small leaks.
I once saw a whole shelf’s worth of reagents evacuated after someone stored solvents next to acids. No one wants to be the reason alarms go off. 2-Piperidinoethanol works best stored tightly sealed, in well-marked bottles, kept cool and dry—away from direct sunlight and far from acids or oxidizers. Labeling sounds basic, but faded markers create confusion. Taking time to update these labels after every use saves a lot of heartache.For cleanup, hazardous waste bins and proper neutralization steps stand between us and accidental exposure. My mentor drilled the point home: “If you can’t say where every leftover drop will go, back away and plan better.”
Every new team member should hear stories—the good, the bad, and the close calls. Safety training takes time, but nothing motivates people more than hearing about what has gone wrong nearby. We drilled emergency eye washes and showers, so even on sleepy days there’s no question what happens next after an accident. Having clear, updated safety sheets nearby helps calm panic, guiding quick action instead of freezing.Fact: The U.S. Occupational Safety and Health Administration lists this compound as an irritant and recommends prompt flushing for skin or eye contact, underlining that it’s never just a routine job.
Safety works best as a shared effort. Habits picked up in school stuck with me, shaping my process every day. Sometimes, it’s the habits—the simple act of double-checking that the hood fan runs, or replacing cracked gloves—that stave off disaster. Giving attention to the little things, respecting labels, and maintaining equipment create a safer place for all.
Anyone working around chemistry, pharmaceuticals, or even certain industrial applications will eventually run into the name 2-Piperidinoethanol. On the page, it shows up as C7H15NO. This string of letters and numbers carries a lot more weight than it first seems. Each part reveals something specific: seven carbon atoms, fifteen hydrogen atoms, one nitrogen atom, and one oxygen atom. Someone who’s mixed chemicals in a lab or studied toxicology recognizes these details as a kind of blueprint, telling you where you might expect reactivity or possible risks.
Through my time teaching undergraduate chemistry, there’s a quote that sticks with me — chemistry is more than memorization, it lives in the connections you make. The piperidine ring in this compound, with its six-membered ring containing one nitrogen, shows up across pharmaceuticals and agrochemicals. That attached ethanol side chain brings a handle for forming more complex molecules or tweaking properties like solubility. Maybe a little technical, but everyday things — painkillers, antihistamines, or even agricultural chemicals — rely on structural tweaks exactly like this.
Missteps involving chemical formulas can trip up even seasoned chemists. I’ve seen research teams fumble with labeling, leading to time wasted and even safety incidents. Knowing the formula C7H15NO means you can track safety sheets, handle spills, and understand which chemical reactions might get out of hand. The formula means immediate context for anyone operating in industry or research: handle with gloves, keep away from strong oxidizers, and double-check your ventilation.
Regulators, too, rely on these formulas. The Environmental Protection Agency and OSHA might not require every worker to know each formula by heart, but information tied to that formula, like toxicity or environmental persistence, drives safety rules. I remember a colleague running a test for freshwater toxicity; knowing the formula let her interpret precisely what was in her water samples, not just trusting a label.
As a chemical, 2-Piperidinoethanol often passes through multiple hands and borders, each step introducing chances for mistakes or even illegal uses. Counterfeit chemicals or incorrect documentation wreak havoc — from logistics delays to health scares. Inspiration comes from the pharmaceutical supply chain, where chemical integrity protects consumers and keeps science honest.
Some misuse results from plain confusion: C7H15NO lines up close to several similarly named chemicals. One mix-up at the supplier and you get unintended reactions or products not fit for purpose. Real cases of manufacturing recalls have revolved around mistakes this simple.
There’s no silver bullet, but greater transparency and consistent education go far. Simple tools, like barcode scanning or chemical authentication apps, help labs and businesses verify what’s on the label. Universities and trade schools can teach not just reaction mechanisms, but the responsibility to double-check. Hard lessons often get learned through small mistakes, but with digital recordkeeping and shared databases, catching errors before they snowball has never been more possible.
Knowing something as seemingly dry as C7H15NO pays real dividends. It’s practical, not just theoretical — a foundation for science, safe handling, and global collaboration.
If you’ve ever handled chemicals like 2-Piperidinoethanol, you know a clear system in your storage area saves headaches and improves safety for everyone. This compound, with its strong odor and hygroscopic tendencies, doesn’t play well with sloppy habits. Leaving it exposed to air long enough will turn a bottle into a sticky mess, sometimes even leading to unsafe conditions.
Years spent working with chemicals have taught me that one shortcut or overlooked protocol can wreak havoc. Despite what labels sometimes suggest, experience reminds us that compounds like 2-Piperidinoethanol will react to everyday moisture and warmth much faster than people think. A quick chat with any lab tech or warehouse worker will reveal more than a few close calls due to misplaced lids or containers left near a heater.
2-Piperidinoethanol reacts with carbon dioxide in air and can absorb water, leading to degradation and possible pressure changes. That’s not just an inconvenience. Contamination can make lab results unreliable or, worse, raise exposure risks to fumes or spills. OSHA data underlines the need for strict storage, since vapor inhalation or skin contact can lead to health problems, ranging from headaches to more serious effects with prolonged exposure. A review from the National Institutes of Health also describes cases where improper storage led to containers bulging outwards or corroding from the inside—all because of small amounts of moisture or acid creeping in.
From what I’ve seen in university labs and small-scale industry settings, the best practice starts with a good airtight bottle, made of materials that don’t react with amines or alcohols. Glass usually works, though oils and dirt from handling sometimes create microscopic cracks. So, workers keep bottles dry and label them clearly with open dates. A dedicated, locked chemical cabinet seated in a cool spot far from any direct sunlight or vents makes a difference; heat speeds up reactions or can cause pressure to build inside containers. In one case, colleagues learned the hard way not to store 2-Piperidinoethanol near acids or oxidizers—a simple mix-up triggered a response that required calling in hazmat specialists.
More often, accidents start with someone putting a container back in the wrong spot. Monitoring who accesses and moves containers not only helps with safety, but also keeps inventory accurate. Reliable inventory management reduces the chances of an “out-of-sight, out-of-mind” incident. Digital tracking systems have paid for themselves many times in my past workplaces, making sure older stock gets used before fresh deliveries get opened.
Chemical storage isn’t just about rules posted on a wall. Real safety depends on repetition and firsthand knowledge. Hands-on training—like practice in using an eyewash station or dealing with a spill kit—reminds everyone that an incident with 2-Piperidinoethanol isn’t a hypothetical scenario. Workers who’ve seen a classmate suffer from chemical burns keep each other honest, double-check bottle caps, and remind colleagues not to cut corners after a long shift.
Solid organizations invest in routine reviews of storage areas. They ask workers to walk through what would happen if a bottle cracked or if fumes started building up in a closet. Fire departments get invited to inspect and offer advice. Even a modest budget supports real safety when lab managers listen to the folks actually handling these compounds every day.
2-Piperidinoethanol doesn’t show up on the supermarket shelf, and most people won’t cross paths with it outside a lab or chemical facility. Workers mixing chemicals, cleaning equipment, or producing specialty compounds bump into it far more than the rest of us. The substance acts as an intermediate in making pharmaceuticals and pesticides—so its reach extends from manufacturing plants right up to products we use daily.
Breathing in 2-Piperidinoethanol can irritate the nose and throat. Splashes to the eyes or skin sting and might even burn, especially after repeated contact. Inhaling higher concentrations creates headaches, dizziness, and nausea, signs that the body’s having a tough time coping. Years ago, I handled chemicals as a research assistant. Even in well-ventilated labs, fumes from similar substances left my coworkers with red eyes and sore throats. That personal memory sticks because safety gear wasn’t always perfect, and it drove home how easily exposure can happen even if protocols look solid on paper.
The National Institute for Occupational Safety and Health (NIOSH) and European Safety authorities both flag 2-Piperidinoethanol as hazardous. They stress the need for gloves, goggles, and local exhaust ventilation. The chemical can absorb through the skin. After a splash, both harm and discomfort follow unless the area gets washed off right away. Chronic exposure, even at low doses, wears on the body—causing dermatitis and persistent coughs for people who deal with it often.
Accidents in chemical plants don’t stay hidden. Spills might reach air or water supplies. A leaky drum in a shipping yard or warehouse can expose unsuspecting workers. A few years back, emergency responders got called out to a spill. Gear kept them safe, but cleanup took hours. Unprotected passersby could have developed severe symptoms. This kind of incident shows how quickly risks grow beyond controlled environments.
Health experts recommend regular monitoring in workplaces where exposure happens. Air sampling, skin checks, and detailed logs spot early signs of trouble. These safeguards matter, because symptoms start small. Employees feeling faint or congested might not connect those signs to a chemical until someone with experience sees the pattern.
Workers need access to personal protective equipment and training on how to use it, not just an occasional seminar. Supervisors enforce the rules, but safety also comes down to familiarity—recognizing a chemical smell, noticing red eyes, and encouraging coworkers to speak up if they feel off. Safety data sheets and proper labeling keep everyone aware of dangers on-site.
Public health agencies push companies to improve ventilation and automation. Machine handling reduces direct contact, helping workers stay healthier in the long run. Some companies switch to less hazardous alternatives if possible, but that usually takes time and investment. Governments can push for stricter reporting and inspections. Investment pays off with fewer sick days and safer communities.
Information about chemical risks changes as research uncovers new facts. Checking regulatory databases and recent studies keeps companies honest and workers informed. People living near plants should ask local officials about emergency planning and what steps get taken if something goes wrong. Even outside direct contact, public knowledge acts as a strong line of defense.
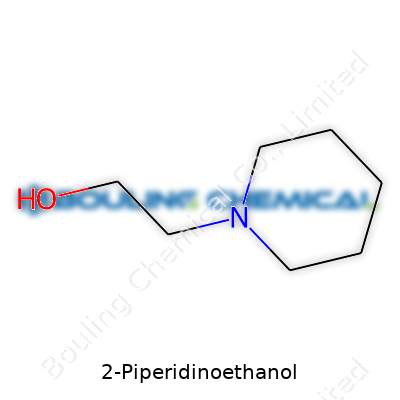
| Names | |
| Preferred IUPAC name | 2-(Piperidin-1-yl)ethan-1-ol |
| Other names |
2-(Piperidin-1-yl)ethanol Piperidine-2-ethanol N-(2-Hydroxyethyl)piperidine 2-Hydroxyethylpiperidine |
| Pronunciation | /tuː paɪˌpɛrɪˈdiːnoʊˌɛθənɒl/ |
| Identifiers | |
| CAS Number | 2003-34-7 |
| Beilstein Reference | 1361171 |
| ChEBI | CHEBI:81315 |
| ChEMBL | CHEMBL78041 |
| ChemSpider | 82574 |
| DrugBank | DB08317 |
| ECHA InfoCard | 100.017.751 |
| EC Number | 202-793-8 |
| Gmelin Reference | Gmelin137022 |
| KEGG | C06396 |
| MeSH | D010888 |
| PubChem CID | 8300 |
| RTECS number | TE1400000 |
| UNII | 2706D5P2NH |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C7H15NO |
| Molar mass | 129.204 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Amine-like |
| Density | 0.978 g/mL at 25 °C |
| Solubility in water | Soluble |
| log P | 0.1 |
| Vapor pressure | 0.01 mmHg (20°C) |
| Acidity (pKa) | 9.64 |
| Basicity (pKb) | 5.65 |
| Magnetic susceptibility (χ) | -64.4e-6 cm³/mol |
| Refractive index (nD) | 1.484 |
| Viscosity | 4.5 mPa·s (20°C) |
| Dipole moment | 2.44 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 252.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -380.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4051.7 kJ/mol |
| Pharmacology | |
| ATC code | C04AX09 |
| Hazards | |
| Main hazards | Causes severe skin burns and eye damage. Harmful if swallowed. Harmful if inhaled. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| Precautionary statements | Precautionary statements: P261, P264, P271, P301+P312, P305+P351+P338, P337+P313, P405, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 102 °C |
| Autoignition temperature | 195 °C (383 °F; 468 K) |
| Lethal dose or concentration | LD50 oral rat 1770 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 1200 mg/kg |
| NIOSH | UU3675000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | REL (Recommended): 5 mg/m3 |
| Related compounds | |
| Related compounds |
Piperidine 2-Morpholinoethanol N-Methyl-2-piperidinoethanol N-Ethyl-2-piperidinoethanol 1-Piperidineethanol |