The story behind 2-(Piperidin-4-Yl)-1H-1,3-Benzodiazole goes back to when chemists began exploring small heterocycles, searching for new chemical scaffolds that could shape drug discovery and material science. Interest in benzodiazole compounds picked up steam during the 20th century. Researchers recognized that fusing varying rings or substituents—like adding a piperidine group at the 4-position—could twist the molecule's biological potential, giving rise to new pharmacophores. Through decades of synthetic research and trial, combinations such as this one cropped up in patent literature and scientific journals. This route followed broader trends in medicinal chemistry, where iterative design led to both hits and misses, but carved a path for what many now see as a cornerstone molecule in the search for central nervous system modulators and more.
2-(Piperidin-4-Yl)-1H-1,3-Benzodiazole falls under the class of heterocyclic organic compounds. The benzodiazole core drives the molecule's rigidity and electronic features, and linking it to a piperidine ring at the fourth position further revises its chemical behavior. Scientists often look at it as a platform for structural analogues, especially for projects that touch on neurotransmitter activity or enzyme inhibition. Its framework allows broad chemical play, making it attractive for medicinal projects, as well as in certain material science applications if the right modification slots into place. Researchers value it both for its direct biological effects and for what modifications can be stacked onto its backbone, supporting ongoing discovery cycles.
This molecule usually hits the bench as a white or off-white solid—crystalline in its purest form, slightly hygroscopic under humid conditions. Chemically, it combines the aromatic stability of benzodiazole with the secondary amine functionality from the piperidine ring. It melts between 120°C and 150°C, sometimes decomposing at the higher end if impure. The compound dissolves well in methanol, ethanol, and DMSO. Water solubility stays moderate—enough for analytical work but not quite in the league of routine biological testing buffers. LogP values typically fall into a range that marks a compound as neither too greasy nor too hydrophilic, which makes it worth a look as a drug candidate. Spectroscopic data—NMR, IR, and MS—provides sharp, readable signals, which eases both quality control and structural verification.
Producers grade 2-(Piperidin-4-Yl)-1H-1,3-Benzodiazole by purity and particulate profile. Analytical specs demand at least 98% purity—measured using HPLC or GC—allowing researchers to move forward with confidence about what’s entering their flasks or test tubes. Standards for heavy metals and residual solvents apply. Labels specify CAS number, molecular weight (217.28 g/mol), storage instructions—often cool, dry, away from strong oxidizing agents—and recommend using desiccators for long-term storage. Typical batch certificates from reliable chemical suppliers back up each shipment with detailed spectrum data. This documentation supports both traceability and compliance, which regulatory bodies crack down on more stringently each year.
Synthetic chemists draw up several routes for making this compound, but a common method starts with nitration of a benzene ring followed by reduction to put the necessary amine functions in place. Diazotization steps then introduce the benzodiazole core, usually under acidic conditions with careful temperature control. N-alkylation with a piperidine derivative at the fourth position comes next, using polar aprotic solvents and a base such as potassium carbonate to promote nucleophilicity where required. Purification involves sequential recrystallization or chromatography, depending on the scale. Each time a chemist makes the compound, tweaks get incorporated to hone yields and minimize the load of side products, reflecting both scientific knowhow and practical lab skills.
2-(Piperidin-4-Yl)-1H-1,3-Benzodiazole’s structure opens doors to chemical tinkering. The nitrogens on the benzodiazole give points for acylation and sulfonation, introducing new groups that can pump up bioactivity or even add fluorescence for imaging studies. The secondary amine on the piperidine ring reacts with alkyl halides or carboxylic acids, producing N-alkyl or amide derivatives. These reactions lead to entire libraries of analogues that medicinal chemists can unleash on high-throughput screening assays. Some groups experiment with selective oxidations or reductive aminations to broaden the range of modifications, trying to eke out either higher receptor selectivity or better metabolic profiles.
This molecule has accumulated a stack of names in the literature and catalogues. Synonyms include 2-(4-Piperidinyl)-1,3-benzodiazole and 4-Piperidinobenzimidazole. Trade listings sometimes alter the order or highlight the benzodiazole or piperidine feature. In NMR or crystallography publications, researchers may abbreviate the backbone to "Pip-BenzDiaz" as shorthand. Keeping these names on hand helps cross-reference between suppliers, scientific papers, and regulatory guidelines, which often trip up studies lacking clarity on substance identity.
Anyone working with 2-(Piperidin-4-Yl)-1H-1,3-Benzodiazole treats it as a standard research chemical, but doesn't ignore basic precautions. Gloves, goggles, and lab coats count as minimum personal protective equipment because benzodiazole derivatives have flagged toxicology risks in the past. Labs ensure good ventilation—ideally a functional fume hood—during weighing and synthesis. MSDS sheets spell out risks of inhalation and skin absorption, noting mild irritant behavior and potential CNS effects if inhaled or ingested in quantity. Safe disposal follows local hazardous waste rules, often treating the substance as cat 5 organic waste. Emergency procedures—eye wash stations, spill kits—stay ready at all times. These steps don't just satisfy audits—they keep researchers healthy and productive in fast-paced settings.
The real excitement with 2-(Piperidin-4-Yl)-1H-1,3-Benzodiazole hits in pharmaceutical research. Medicinal chemists lean on its scaffold to craft molecules that target serotonin, dopamine, and other neurotransmitter systems. The compound’s shape lets it act as a molecular probe, helping researchers map receptor-ligand interactions. Some evidence ties benzodiazole analogues with anxiolytic, anti-inflammatory, or even anticancer effects. Analytical labs value it as a reference material for chromatographic method development. Beyond health sciences, materials scientists poke at its aromatic backbone when testing new conductive polymers or molecular sensors. These uses spark collaborations across disciplines, showing the molecule’s versatility.
Focused research drives most innovation tied to this molecule. In recent years, big data and high-throughput screening have sped up analogue design—labs generate hundreds of derivatives, feeding results into machine learning models. This approach narrows down active candidates ahead of animal or clinical testing. Many research groups, especially in Europe and North America, share synthetic pathways and biological data through open repositories, building a larger conversation around SAR (structure-activity relationship) trends. This collective push chips away at old silos, knitting together basic chemistry and applied pharmacology.
Toxicologists pay close attention. Early screens rely on in vitro models to spot mutagenicity and cytotoxicity. Reports suggest that, like many heterocycles, this compound signals only mild cellular toxicity at low concentrations, but higher doses impair mitochondrial function in some tissues. Animal studies probe for neurotoxicity and potential off-target effects. Repeated challenges reveal dose-dependent CNS depression in rodents, which leads to safety margins lower than for some related scaffolds. Labs run metabolic profiling using human enzymes to flag dangerous metabolites. Regulatory guidance recommends additional genotoxicity and long-term carcinogenicity tests before thinking about anything outside early phase trials.
2-(Piperidin-4-Yl)-1H-1,3-Benzodiazole’s future looks tied to multidisciplinary research. Drug designers plot new analogues using AI-driven algorithms, searching for profiles that dodge old toxicity traps but hit clinical endpoints in depression, anxiety, or oncological pathways. Advances in computational chemistry promise better predictive models for both binding and off-target effects. Startups experimenting with wearable biosensors tap into benzodiazole frameworks for sensing components, driven by progress on miniaturization and real-time analysis. Pharmaceutical and materials science communities need to keep talking—sharing data, pooling resources, and stepping outside comfort zones. Progress won’t just rely on chemistry—biologists, engineers, and data scientists will help uncover uses few could imagine a decade back.
2-(Piperidin-4-Yl)-1H-1,3-Benzodiazole catches the attention of researchers who keep their focus on therapeutic discovery. Not because it shows up in the headlines but because it forms a solid backbone for small-molecule drug scaffolds. Many labs count on this compound when they screen for new inhibitors or blockers that target central systems in the body, like the receptors involved in mood, memory, and pain control. As a medicinal chemist, you soon learn that some molecular shapes tend to play well with tricky targets like GABA, serotonin, or dopamine receptors—and the benzodiazole ring coupled with the piperidine segment brings just the right strengths.
Researchers in the search for anti-epileptics, anti-anxiety drugs, and treatments for neurodegenerative conditions, often turn to these frameworks as prototypes. The reason lies in the unique ability of these rings to both cross biological barriers and show precise activity at nerve cells. Years of published work prove that minor changes at the benzodiazole or the piperidine side can tip a molecule between useless and groundbreaking. This bread-and-butter chemistry supports hard-won progress toward safer, better neuroactive drugs.
The labs that focus on cell biology and pharmacology turn 2-(Piperidin-4-Yl)-1H-1,3-Benzodiazole into valuable chemical probes. These small molecules help answer big questions about how proteins behave inside live cells. For example, if you want to watch a specific receptor’s response, modifying this compound with a fluorescent tag or an imaging unit creates a tracer. It becomes much easier to visualize receptor binding, track protein movement, and build new assays for high-throughput drug screens. Using these probes leads to papers that map out new cell pathways or uncover drug resistance in cancer cells, saving months in experimental time.
Benzodiazole cores have earned a place in the synthetic chemist’s toolbox. 2-(Piperidin-4-Yl)-1H-1,3-Benzodiazole’s popularity comes from the ease with which you can attach other groups and build chemical diversity. Stepwise changes allow for fine tuning of pharmacological properties—a critical part of structure-activity relationship (SAR) studies. I’ve seen chemists in medicinal and material science settings use this approach to generate entire libraries of analogues. They’re looking for that one change that gives either a new drug lead or a new property, like better solubility in different environments.
Particularly in early drug discovery, speed matters. Having a ready route to these benzodiazoyl derivatives cuts weeks or months from the path to a testable candidate. Many early leads die out due to metabolic instability or poor absorption. Using frameworks like this one, you get more control over those problems with less backtracking.
One real concern for anyone working with these molecules is safety—both in the lab and in the environment after their use. Some benzodiazole derivatives show persistent toxicity, building up where you least expect it. We’ve seen regulatory hurdles rise for similar scaffolds in other settings, so chemists now aim for safer, more “biodegradable” variations and regularly screen for unwanted side effects early in the project. Open sharing of safety and toxicity data could close gaps faster, preventing wasted time chasing unsafe or impractical candidates.
Overall, compounds like 2-(Piperidin-4-Yl)-1H-1,3-Benzodiazole have reshaped modern chemistry’s approach to both discovery and problem-solving. Careful use, constant attention to impact, and creative collaboration stand as the best tools for pushing these applications further—turning basic building blocks into something that truly improves human health.
Questions about chemical purity show up nearly every day in labs and industries that handle pharmaceuticals, food, electronics, and anything that relies on exact measurements. People see purity percentages stamped on containers, often trusting the number without digging deeper into what it means. For someone like me who’s had to troubleshoot both science fair mishaps and high-end instrument failures, chemical purity is more than a number—it can make or break results.
A product with 98.5% purity isn’t simply a bottle filled with nearly all one substance. It still contains a slice—with the rest made up of other chemicals or trace materials. Manufacturers lean on tools like chromatography, mass spectrometry, and titration to measure these fractions. Still, the number on the label comes with conditions: what substances they tested for, which ones actually matter to the use, and whether the report captures the right picture. The most important information hides in the impurities—their type, not only how much.
Take a bottle of sodium chloride. Table salt in the kitchen rarely matches lab-grade salt. In a lab, if you’re isolating a reaction that’s sensitive to even a hint of magnesium, a small impurity could throw readings off. One winter, I tried to help a neighbor de-ice his drive, and the “pure” salt left behind an unexpected purple streak: manganese in the mix. Small impurities can change results, color, taste, and even safety.
Pharmaceuticals need strict attention to purity. Tiny deviations—think parts per billion—can mean the difference between safe treatment and harmful side effects. In my former lab days, regulations from the FDA required us to document every contaminant that crossed the line, not just the big ones. The wrong impurity at a low percentage could send an entire batch of medicine back to the start, costing time and trust.
No two products carry the same meaning for “pure.” Pure water for injection gets tested for endotoxins, bacteria, and heavy metals, not just the absence of other chemicals. A pure solvent for electronics needs near-zero water content and a specific profile of contaminants avoided.
Companies in the market for raw materials often ask for a certificate of analysis—not just the purity percentage, but a full list of detected impurities and the methods used to find them. If a supplier’s analysis misses one test or doesn’t tell you about an unusual additive, everything made with that batch runs a risk.
At its core, purity matters because people trust the products they use every day. Kids expect candy coloring to be safe, farmers trust fertilizers to feed crops and not the weeds, doctors count on pain relievers to work as promised. Manufacturers and customers can help each other by pushing for test results that show both the big picture and the small print.
Labs can run more frequent spot checks, invest in better testing gear, or partner with independent groups for audits. Open sharing of test data gives everyone—from the person mixing chemicals in a high school to the engineer building a spacecraft—a better shot at success. The real value isn’t just in the number but in knowing what’s in your bottle and how it will work in the real world.
Anyone who’s worked in a lab knows how quickly things can sour if chemicals aren’t treated with respect. 2-(Piperidin-4-Yl)-1H-1,3-Benzodiazole doesn’t exactly roll off the tongue, but its handling and storage speak volumes about lab safety and professionalism. I’ve spent plenty of hours labeling containers, double-checking seals, and talking through safety data sheets with new researchers. The point always hits home: even routine chemicals can cause headaches—sometimes literally—if they’re not tucked away correctly.
People often overcomplicate storage protocols, putting their trust in institutional memory or a hope that nothing goes wrong. That approach rarely works for long. For 2-(Piperidin-4-Yl)-1H-1,3-Benzodiazole, a sturdy amber glass bottle with a tight seal ends up working better than anything flashy. Light plays tricks on many compounds. Over time, exposure to sunlight or even bright overhead lamps causes them to degrade, change composition, or lose effectiveness. Storing this benzodiazole in a dark, temperature-controlled cabinet thwarts these problems before they start.
Temperature swings trigger anxiety in every chemist. Too much heat or a cold snap, and you risk more than ruined chemicals—you face unpredictable reactions and data that don’t add up. Refrigeration usually keeps the chemical in a stable state, but it pays off to read the product’s safety data sheet and look for the recommended range. Many years in labs have taught me that assuming “room temp is fine” often leads to calls with chemical suppliers after spoiled batches.
Organization can save hours, prevent lost work, and avoid safety scares. Skipping on documenting lot numbers, opening dates, or storage history creates a hassle for the next person in line—sometimes me, sometimes someone less experienced. Every bottle or vial deserves a clear label. Add the date it goes on the shelf and who opened it. If something goes wrong, that log bridges the gap between confusion and clarity.
Shelving systems in chemical storage rooms need attention. Too many labs relegate dangerous or reactive substances to top shelves “out of reach.” This choice often puts the most hazardous compounds where they can fall and break. Lower, secured shelves reduce risk. Anyone reaching for 2-(Piperidin-4-Yl)-1H-1,3-Benzodiazole should encounter labeled, closed containers on stable ground—never balanced precariously above eye level.
Gloves aren’t optional with specialty benzodiazole analogues. Even limited exposure causes skin irritation or more serious reactions, depending on concentration. Fume hoods exist for more than show. I make a habit of handling anything with a piperidine group under a hood, away from snacks or drinks, and well away from accidental contamination of communal surfaces.
Spill kits and emergency protocols should be within arm’s reach, not buried behind stockpiles of printer paper and cardboard boxes. Rapid cleanup, supported by clear protocols, means small accidents stay small. In days gone by, I watched a minor benzodiazole spill escalate simply because equipment wasn’t handy. That experience stuck with me longer than the acrid odor did.
Safe chemical storage is only part of the story. At the end of its useful life, 2-(Piperidin-4-Yl)-1H-1,3-Benzodiazole deserves proper disposal. Dumping anything down the drain puts both people and the environment at risk. Most suppliers offer guidelines for neutralization or pickup; some labs run regular hazardous waste collections. The key is keeping waste streams from building up and attracting problems—either regulatory or practical.
Chemical stewardship has changed over the years, moving away from casual storage toward a more mindful, evidence-based approach. Thanks to solid documentation, good habits, and regular training, labs run smoother. Chemicals like 2-(Piperidin-4-Yl)-1H-1,3-Benzodiazole earn their keep by producing reliable results and, more importantly, by never surprising anyone who opens the supply cabinet.
Asking if a compound comes in bulk seems like a yes-or-no question, but anyone who has spent time sourcing chemicals learns how complicated things get. In the lab, tiny bottles feel common. Scale up to full-scale manufacturing, and it’s a different story. A friend in pharmaceuticals once hit a wall searching for a kilo of an odd-ball intermediate. The catalog listed it, but the supplier needed six months and triple the price for her quantity. That frustration pops up across research, agriculture, and food processing.
A healthy supply chain answers demand with scale. Global markets set raw material prices, and there’s no guarantee that something easy to buy in grams goes by the drum. Specialty chemicals with tight regulations face even thicker roadblocks — customs, permits, and shelf-life challenges create further slowdowns and costs.
Bulk availability can make or break a project. Pharmaceutical plants often plan years ahead, just to secure contracts for needed chemicals. The whole drug launch depends on having tons of a starting material at the right time. In agriculture, supply gaps mean missing a planting window or, worse, facing crop loss from an outbreak.
Many companies have risk teams keeping close tabs on how much key materials get produced every year, who controls the supply, and what countries are affected by conflict or environmental issues. The COVID-19 pandemic highlighted this problem: breakdowns in transport delayed shipments everywhere. Even common reagents vanished from shelves as countries hoarded supplies for local use.
Public chemical catalogs paint an incomplete picture. A compound appearing “in stock” might mean a few grams, or sometimes just the promise that it could be made to order. And “bulk” means different things to different people—a university might call a few kilos “large scale,” while a fertilizer producer thinks in shipping containers.
Without clear communication, buyers spin their wheels asking for price quotes on orders suppliers can’t really fill. I’ve seen tech transfer efforts bog down at this exact stage: the supplier commits in small print, but flakes out at delivery or misses quality specs for larger orders.
It’s not just about quantity. Researchers moving to pilot scale learn quickly that synthesis routes that work for a test tube might falter at 50 kilos. Waste handling, purity requirements, and worker safety turn easy recipes into obstacles in a factory. Bulk purchase raises questions about batch-to-batch consistency, and environmental impact grows.
Farmers and industry buyers also give thought to greener production methods, current Good Manufacturing Practices (GMP), and local sourcing. Some large suppliers invested in regional plants just to avoid headaches over customs and tariffs.
Building real transparency would help everyone, from researchers to engineers. Suppliers could update catalogs to flag compounds with genuine bulk supply and set realistic timelines. Buyers could collaborate with producers earlier in R&D to plan for scale. Communication, honesty, and flexibility drive real progress here.
Sourcing compounds in bulk is not simply a matter of demand but a test of logistics, planning, and trust between buyers and suppliers. For anyone planning a breakthrough—or just the next batch—asking about availability early can mean dodging a costly delay. Industry thrives on knowledge and relationships as much as chemistry.
People don’t walk around holding encyclopedias on chemical safety, yet many use household cleaners, paints, lawn products, or industrial supplies every day. Questions about what’s actually inside those bottles and how to handle them rarely come up—until something goes wrong. I’ve experienced confusion at the hardware store, staring at a jug with hazard symbols but zero practical advice beyond, “Use in well-ventilated area.” The need for real, understandable safety data and handling steps hits home quickly once a splash lands on skin or pungent fumes invade the room.
The tiny print on the back of most labels tries to cover legal ground, but average folks don’t gain much from it. Safety Data Sheets (SDS) end up scattered online, often outdated or locked behind registration pages. According to the U.S. Occupational Safety and Health Administration (OSHA), over 2 million workers face exposure to hazardous chemicals that can cause serious health issues. For the general public, these risks multiply when information hides behind jargon or web forms.
Years ago, a co-worker got splashed by a strong cleaner and fumbled through a heap of paperwork to find out what to do. This real-world scramble could have been avoided by accessible, plain-language guidance. Instead, workers and families alike deal with the hazards while struggling to make sense of complicated documents.
Safety isn’t served by secrecy or legal speak. It starts with companies offering clear instructions about what a product contains, how to use it safely, and what to do if something goes wrong. For all the talk about innovation, too many brands still hide the important details in layered PDFs or shrink the text so small it feels like a dare.
Accidents happen not because people act carelessly, but because they can't find or understand the right steps. The most common errors, like mixing incompatible chemicals or improper storage, stem from poor guidance rather than obvious recklessness. According to the American Association of Poison Control Centers, over 95% of accidental chemical exposures happen at home. So this isn’t just a workplace problem—it's relevant to everyone.
Some companies treat safety as an afterthought, but others prove it can be done better. Placing QR codes on labels that link directly to readable, updated SDS files takes little effort now but brings big benefits. Posting videos and infographics showing best practices instead of expecting every user to comb through technical sheets builds trust and prevents injuries.
Retailers and regulators also play an important part by setting minimum standards for what safety data needs to look like. The European Union’s REACH regulations forced many companies to improve access to chemical information. In the U.S., pressure from labor unions and consumer advocates has pushed for broader availability of straightforward safety resources.
A decade ago, most shoppers didn’t care what went into cleaners or sprays. Today, people want answers. As someone who’s scrambled mid-emergency looking for answers in the fine print, I’ve learned that accessible safety data isn’t just a legal obligation—it's a public good. Smart companies and communities make room at the table for everyone by offering instructions that don’t require a chemistry degree. That’s how we avoid disasters before they happen and help people feel confident, not cautious, about the products they use every day.
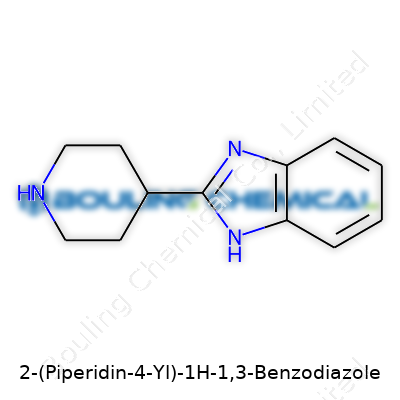
| Names | |
| Preferred IUPAC name | 2-(Piperidin-4-yl)-1H-benzimidazole |
| Other names |
1H-Benzimidazole, 2-(4-piperidinyl)- 2-(4-Piperidinyl)-1H-benzimidazole 2-(4-Piperidyl)benzimidazole |
| Pronunciation | /tuː paɪˈpɪrɪdɪn fɔːr aɪl wʌn eɪtʃ wʌn θriː bɛnˈzəʊdaɪˌæzəʊl/ |
| Identifiers | |
| CAS Number | 846589-98-8 |
| 3D model (JSmol) | `load =C1CN(CCN1)c2ncc3ccccc3n2` |
| Beilstein Reference | 1988347 |
| ChEBI | CHEBI:72803 |
| ChEMBL | CHEMBL2105656 |
| ChemSpider | 23865166 |
| DrugBank | DB07241 |
| ECHA InfoCard | 35c95b34-815c-44bb-b0d7-54c1fac1e0a1 |
| EC Number | EC 619-568-8 |
| Gmelin Reference | 834957 |
| KEGG | C14768 |
| MeSH | D08.811.277.040.352.650.146.300 |
| PubChem CID | 22708924 |
| RTECS number | HP1420000 |
| UNII | 3W1183Z38Z |
| CompTox Dashboard (EPA) | DTXSID3020169 |
| Properties | |
| Chemical formula | C11H15N3 |
| Molar mass | 219.29 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.22 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.72 |
| Vapor pressure | 0.0000133 mmHg at 25°C |
| Acidity (pKa) | 7.7 |
| Basicity (pKb) | 2.99 |
| Magnetic susceptibility (χ) | -0.0000125 |
| Refractive index (nD) | 1.645 |
| Dipole moment | 3.52 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 251.7 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | N05BE03 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | > 141.6 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 873 mg/kg (rat, oral) |
| NIOSH | Not listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg |
| Related compounds | |
| Related compounds |
1H-Benzimidazole 2-Substituted benzimidazoles 4-Piperidinyl benzimidazole Piperidine Benzimidazole derivatives N-alkyl benzimidazoles |