Communities of chemists first took notice of piperidine rings in the 19th century, and the search for modifications began soon after. When ethanol branches attached themselves to this scaffold, a host of new molecules became possible, including 2-piperidin-2-ylethanol. At first, the tuning of this compound trailed behind more popular amine and alcohol derivatives. Academic research in the mid-20th century shifted, and interest in heterocyclic frameworks led to a spike in reported syntheses. Early records show researchers in medicinal chemistry labs tinkering with piperidine-based alcohols. This interest now continues, as industrial and research laboratories pursue new functionalities and sustainable syntheses, using 2-piperidin-2-ylethanol as a stepping stone.
2-Piperidin-2-ylethanol combines two functional elements: a secondary amine from the piperidine ring and a primary alcohol. Manufacturers most often supply it as a colorless to pale yellow liquid or solid, depending on purity and storage temperature. Researchers prize its reactivity, as the combination of alcohol and nitrogen in its backbone opens up diverse downstream chemical modifications. The compound is often used as an intermediate for pharmaceuticals, agrochemicals, and specialty catalysts. Its structure shapes its role in many chemical strategies, as few other molecules combine piperidine and hydroxyethyl so seamlessly.
The compound melts around room temperature, though the crystalline form sometimes requires careful chilling. The molecular weight hovers just below 130 g/mol, and the boiling point tends to fall between 230 and 240°C at standard pressure, which means you can purify it by careful distillation but risk decomposition if you rush the process. In terms of solubility, 2-piperidin-2-ylethanol dissolves readily in water and most organic solvents, thanks to its alcohol and amine groups. It behaves as a mild base due to the nitrogen atom, giving it moderate reactivity with acids and electrophiles. It does not react violently with air or water, but its basicity and alcohol function create opportunity for multiple downstream transformations.
Producers list 2-piperidin-2-ylethanol with CAS number 33486-14-9. Regulatory labeling typically details hazard warnings around skin and eye irritation, as well as possible environmental persistence. Purity for technical and research use often exceeds 98%. The SDS will describe measures for personal protection and engineered ventilation, and the label emphasizes the importance of proper storage, particularly excluding strong oxidizers or acids. Some suppliers polymerize impurities more slowly by using amber glass or nitrogen blankets for long-term storage, and larger containers bear secondary containment and spill instructions. Batch traceability marks and expiration dates assure users the material has not degraded.
Manufacturers commonly synthesize 2-piperidin-2-ylethanol through ring-closure reactions of appropriate amino-alcohols or by the reduction of keto intermediates. One approach combines 2-piperidinone with ethylene oxide under controlled alkaline conditions, creating high yields but demanding careful pH management and slow addition to avoid runaway reactions. Another route uses Grignard reagents or organolithiums to introduce the ethanol group onto activated piperidine rings, though handling these intermediates requires experience with air-sensitive chemistry. Reduction strategies start with piperidin-2-one and reduce it with hydride donors in alcohol solvents; this method is receptive to large-scale implementation. Across all routes, product purification by distillation or crystallization assures the quality needed for further synthetic steps.
Having a free alcohol and a secondary amine makes 2-piperidin-2-ylethanol versatile. Alkylation, acylation, and etherification typically modify the alcohol, adding bulk or adjusting polarity. Protection-deprotection strategies use silyl or benzyl groups to block either end, for selective functionalization. Researchers exploit the amine for reductive amination or to create amide linkages, particularly in pharmaceutical intermediate syntheses. Oxidation of the alcohol produces aldehydes or carboxylic acids, opening routes to even more functionalized substructures. Pairing these traits, the molecule can help build complex polycyclic compounds, or link multiple functional domains in bioactive agents or even polymer monomers.
In academic and commercial circles, you’ll find 2-piperidin-2-ylethanol also described as 2-(2-Hydroxyethyl)piperidine or simply piperidinylethanol. European and Asian catalogs might list it as 2-hydroxyethylpiperidine. Despite small naming differences, reference to CAS 33486-14-9 usually clears up confusion. Some chemical databases use older naming formats, such as β-hydroxyethylpiperidine, and misspellings abound due to the odd double “2” in its preferred IUPAC name. For product identification, cross-referencing with chemical structure and registry number remains best practice.
Labs and process plants treat 2-piperidin-2-ylethanol with the respect typical of low-volatility amines. Gloves and eye protection form the basic outfit, as the compound stings eyes and can cause dermatitis with repeated skin exposure. Ventilation proves essential, not just for handling dust or vapor but for air-sensitive reactions that involve strong bases or acids. Standard operating protocols call for quick cleanup of spills, absorption with inert media, and disposal as hazardous organic waste. Fire risk stays low, but in an emergency, CO2 or foam extinguishers take priority over water. Long-term stability depends on cool, dry storage and the exclusion of reactive materials. Occupational exposure limits are not tightly regulated, but prudent management minimizes handling time and ensures quick access to MSDS instructions.
Pharmaceutical development leads the demand, as 2-piperidin-2-ylethanol steps in as both a building block and a chain extender for more complex heterocycles. Its role in agrochemical synthesis comes close behind, where it helps create chiral ligands or pesticide intermediates. Synthetic chemists value it for the flexibility of its alcohol group; linker chemistry, functionalization, and development of bioactive molecules form the main pathways. In catalysis, the molecule sometimes anchors ligands to solid supports or modifies chelating backbones, increasing selectivity and process efficiency. While less common, it pops up in polymer chemistry as a monomer or additive, adjusting chain properties or hydrophilicity for advanced coatings and resins. Its compatibility with fine chemical production makes it a staple in pilot and bench-scale routes for new material synthesis.
Recent research on 2-piperidin-2-ylethanol explores bioactivity, chiral pool exploitation, and regioselective functionalization. Academic and industrial groups chase after efficient, green synthesis protocols to reduce energy and hazardous byproducts, including biocatalytic routes with whole cells or tailored enzymes. Some groups publish studies on asymmetric modifications to access enantiomerically pure forms for pharmaceuticals. Lab groups develop high-throughput screening routines, where the ethanol and piperidine moieties allow combinatorial methods for drug candidate libraries. Analytical research includes detailed NMR, mass spectrometry, and IR studies to track purity and map side product profiles, as impurities or stereoisomers can have significant downstream effects.
Published animal studies suggest mild to moderate toxicity profiles, typical of secondary amines and small alcohols. Acute exposure in rodents leads to irritant effects but rarely fatal poisoning, though repeated dosing does raise liver enzyme levels or mild neurotoxicity. No legitimate evidence yet links 2-piperidin-2-ylethanol to mutagenicity or carcinogenicity, but the field could benefit from more studies, especially with repeated low-dose exposures and bioaccumulation risks. Chemical safety committees require more granular chronic toxicity data before allowing wider use in consumer products. Waste management takes these risks seriously, ensuring destruction of the molecule before environmental release.
Interest in 2-piperidin-2-ylethanol looks set to grow, as its core structure fits with shifting trends in drug discovery, catalysis, and material science. Researchers look for sustainable syntheses, aiming for biobased feedstocks or enzymatic routes to cut out hazardous reagents and lower overall emissions. If greener production matures and costs drop, large-volume technical uses in polymer and coatings technology could open up. Regulatory landscapes change fast, so ongoing work on toxicology and environmental fate finds itself on the front lines, ensuring this molecule doesn’t get left out as greener alternatives rise. Continued innovation in selective functionalization will expand its toolkit for custom molecules, giving future chemists both options and insight.
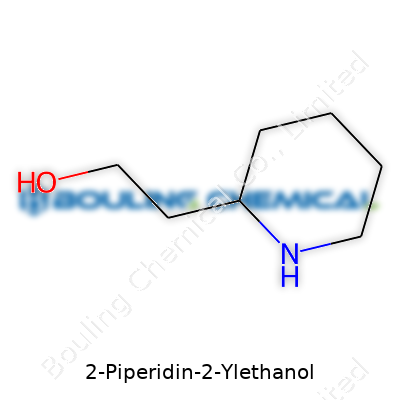
2-Piperidin-2-ylethanol grabs my attention every time I work in the lab or thumb through chemical catalogs. Picture a six-membered ring, made entirely of carbons and a single nitrogen, called piperidine. An ethanol group – that’s a two-carbon side chain with a terminal alcohol – clings to the ring at its second position. That placement matters: it defines the name and the molecule’s core function in the hands of medicinal chemists.
The ring in piperidine gives this compound a backbone loaded with possibilities. Five carbons, one nitrogen. At carbon 2, a -CH2-CH2OH shoots off. This side chain changes the usual behavior compared to plain piperidine. Chemically, we write the formula as C7H15NO. Count seven carbons, fifteen hydrogens, and one each of nitrogen and oxygen. With this setup, a unique blend of basicity and alcohol reactivity sits in a single package. In medicine and materials science, that mix matters.
In my experience, small tweaks to a structure, like tossing an ethanol group onto piperidine, often flip the script. These adjustments dramatically change solubility, shape, the way a drug binds, and even how it enters a cell. Piperidine itself stars in pharmaceuticals for treating mental health conditions and in antispasmodics. A subtle push, like that ethanol unit, offers a new tool for chemists. It brings extra hydrogen bonds, opening up new pockets for interaction with proteins.
There’s a reason so many drugs rely on piperidine rings: the structure carries a balance of flexibility, stability, and reactivity. Tacking on a -CH2CH2OH group lets compound developers fine-tune both water solubility and the position of active groups. These seemingly small shifts can help a drug last longer in the bloodstream or sneak past cellular barriers. In my own work, a molecular modification like this can mean fewer side effects or greater impact at lower doses.
This molecule’s detail isn’t just academic. Unsafe shortcuts often pop up in small-scale labs racing to produce new compounds or imitating market drugs. Detailed knowledge helps ensure safe handling and clear communication, especially for those scaling up for trials or commercial runs. Understanding every atom’s position helps anticipate risk and optimize outcomes.
The shape of 2-piperidin-2-ylethanol also hints at its place in the wider sweep of drug design and specialty chemicals. With one nitrogen ready for basic reactions, and a primary alcohol group ripe for further modifications, labs often use this core to create bigger, more complex compounds. It’s a stepping stone, often underappreciated, to new therapies or advanced materials that could change the way we live.
I believe chemistry communities, both academic and industrial, owe it to themselves to treat even small derivatives seriously. Proper labeling, risk awareness, and data transparency protect not just researchers, but anyone downstream. Open-source safety data, alongside details about metabolic breakdown, help researchers and doctors avoid unwelcome surprises. Partnerships between labs, universities, and industry groups keep innovation honest and informed.
Building a deeper understanding of structures like 2-piperidin-2-ylethanol isn’t just chemical trivia. It’s part of a commitment to safer research, better drugs, and real progress. Cutting corners on knowledge leaves everyone at risk, while taking the time to see how one ring and a side-chain can shape the world pays off in discovery and trust.
Anyone who’s spent time in a laboratory—especially in organic synthesis or pharmaceutical research—probably recognizes the significance of uncommon building blocks like 2-Piperidin-2-Ylethanol. There’s no flood of fame around this compound, but it manages to sneak into a surprising number of projects. Its piperidine ring puts it in good company with other key intermediates, lending structure and flexibility in a variety of different domains.
Pharmaceutical research keeps this molecule busy. Its basic skeleton helps medicinal chemists tweak properties in early drug design. Modifying the piperidine ring with the ethanol side chain opens doorways to new analogues and variations. Drug discovery folks love these tweaks: they can change how a molecule binds to an enzyme or alter its solubility. In the process of hunting for new drugs, fine-tuning the shape or chemical behavior of a candidate molecule sometimes means starting with something like 2-Piperidin-2-Ylethanol. In a typical medicinal chemistry program, chemists often create dozens, sometimes hundreds, of analogues; the side chains and backbone changes matter a lot. This molecule sits right in that crossroads, ready to deliver those essential variations.
2-Piperidin-2-Ylethanol shows up in the agrochemical world too. As an intermediate, it helps manufacture compounds used to protect crops or promote growth. The agriculture sector depends on safe, effective products that resist breakdown in the field but don't linger too long in the food chain. By introducing its structure into certain molecules, researchers can balance breakdown rates and biological activity. In my own experience, working with formulation teams, we’d sometimes incorporate piperidine derivatives to improve the stability or targeting of new plant treatments. The ethanol functionality on the ring often makes it easier to connect with other chemical fragments, speeding up development.
Material scientists haven’t ignored this compound either. There’s growing interest in specialty polymers built for electronics or biomedical applications. Sometimes, adding a nitrogen ring like a piperidine can boost flexibility or increase resistance against degradation. The extra ethanol group lets scientists attach the ring to larger structures or react with functional groups along a polymer chain. I remember a project focused on antistatic coatings where this kind of backbone brought about subtle but noticeable improvements in performance. Working with polymer blends, chemists used 2-Piperidin-2-Ylethanol to introduce specific sites for further modification, which made it easier to fine-tune properties for high-tech applications.
One can’t ignore the importance of research-scale projects. Often, what happens on the bench gets overlooked by large-scale manufacturing, but high-level research seeds tomorrow’s breakthroughs. 2-Piperidin-2-Ylethanol often acts as a key step in assembling more complex molecules custom-made in research settings. Scientists use it to explore new molecular frameworks, synthesize chiral compounds, and even study reaction mechanisms.
The chemistry world constantly faces the push to develop more sustainable and less toxic processes. This molecule, like many others, brings challenges along with its advantages. Responsible handling matters. Training in safe laboratory practices keeps risks in check. Manufacturers and researchers benefit by investing in green chemistry approaches—think fewer waste byproducts, safer reagents, or recycling solvents. Collaborations among academia, industry, and regulatory groups help shape guidelines that keep both people and the environment safe. Continuous improvement in how people use and manage chemical intermediates like 2-Piperidin-2-Ylethanol will keep its contributions both valuable and responsible in the years ahead.
I’ve spent enough years around lab benches and safety datasheets to know one thing: every chemical deserves respect, especially intermediates and reagents like 2-Piperidin-2-ylethanol. This molecule, used in specialty synthesis or pharmaceutical research, shows up every so often in research environments. When you hear about a piperidine derivative, you don’t shrug off safety. That’s just the way the chemistry world keeps people healthy and out of trouble.
The information on 2-Piperidin-2-ylethanol’s toxicity sits a little sparse compared to mainstream solvents or household chemicals. Most chemists will immediately look for acute oral, dermal, and inhalation toxicity. Generally, compounds related to piperidine often cause skin and respiratory irritation and can punch hard if handled carelessly. In similar substances, prolonged or repeated exposure has sometimes led to headaches, dizziness, or worse. Industry safety data sheets (SDS) often flag these molecules with hazard statements like "may cause respiratory irritation" or "harmful if swallowed," usually backed up with rabbit or rodent studies that leave zero doubts in my mind.
Liquid or solid, 2-Piperidin-2-ylethanol won’t announce itself with a strong smell or color. Accidental splashes could slip by unnoticed if personal protective equipment gets skipped. Once, during a late-night cleanup, I saw a colleague shrug off a glove stain—only to deal with a rash hours later. Common sense in the lab means gloves, goggles, and fume hoods.
The real danger often comes from short-cuts: Not securing lids, working outside proper ventilation, or pipetting by mouth (don’t!). Lab safety isn’t about paranoia, but about seeing the value in checklists and staying meticulous. I read a report of skin absorption cases that led to odd numbness or local irritation. You don’t want surprises when skin contact gets involved.
I always check the flash point. 2-Piperidin-2-ylethanol sits in the mid-range: not the most combustible, but you keep it far from open flame. Water-reactivity stays low, but mixing with strong oxidizers or acids brings unpredictable reactions, and that never ends well. Labeling and isolation from incompatible materials saves time and worry. In practice, I’ve seen firefighters hesitate over unmarked reagent bottles, wasting precious minutes in emergencies. Clear hazard labeling helps everyone react fast and stays in line with chemical regulations.
Basic precautions win the safety game: fresh gloves, disposable pipette tips, proper waste containers, good airflow. Relying on old habits can be a problem—I once walked into a lab where outdated spill kits and faded signage were left untouched. Quick refreshers for everyone in the lab keep accidents from turning serious. Management support helps a lot: up-to-date training, clear accident reporting lines, strong supply of fresh PPE.
In the bigger picture, hazard communication law demands proper risk assessment for every chemical, including 2-Piperidin-2-ylethanol. Updating inventory records and enforcing safety protocols pays off faster than you might think. Fact: U.S. occupational regulations require chemicals to have a Safety Data Sheet, and staff must know what to do if a spill or exposure happens.
If a process lets you swap out a more hazardous piperidinyl compound for something safer, that’s worth a look. Finding alternatives can sometimes take time or extra testing, but sometimes it’s the only way to keep people safe in the long term. Working together with industrial hygienists or chemical engineers brings up new protocols and safer pathways, making the whole lab environment healthier and better-prepared for the unexpected.
2-Piperidin-2-ylethanol pops up in a variety of chemical toolkits, especially where intermediates drive new product development. Checking purity isn’t just box-ticking; a slight move in the numbers might throw off research or manufacturing, lead to unpredictable reactions, or spike costs down the line. Lab suppliers usually list purity at 97% to 99%, with HPLC or GC analysis backing their claims. Lower figures crop up sometimes, but those require extra scrutiny since even trace impurities can change everything about the result, especially if your work depends on sensitive syntheses or if you’re on a tight regulatory leash.
Many researchers order higher-purity grades, not because marketing says it matters, but because consistent results let them sleep at night. On my own workbench, a difference of 1% in a precursor like this sometimes made or broke a run—particularly in pharma or agrochemical testing, where impurities sneak into final analysis and muddy interpretation. Purity data doesn’t fit a set-it-and-forget-it mold. Batch numbers and certificates of analysis tell a story: where it came from, how it was tested, and whether anyone can stand behind the numbers printed on the sheet. Skipping this step invites headaches, especially if tests start failing or a regulatory inspector comes calling.
Quality chemicals rarely ship in bulk without good reason. Most suppliers bottle 2-piperidin-2-ylethanol in amber glass containers. This isn’t just for show. The compound’s stability improves in opaque bottles, and glass avoids chemical interactions that plastics might invite, especially if the bottle needs to sit for a few months before opening. Typical lab packaging ranges from 5 grams up to 500 grams—much more than that, and you’re likely talking to an industrial chemist, not a bench scientist.
Every bottle I’ve handled came with detailed labeling: batch number, storage suggestions (room temperature, sealed, sometimes with a desiccant for long storage), and a hazard chart. Safety data sheets often arrive tucked in or emailed after purchase. Suppliers with a science-first focus print clear expiration dates and warnings if refrigeration beats room temperature. I learned quickly to keep two eyes open for that sort of advice, since even the best chemicals degrade if forgotten on a warm shelf.
Problems still come up. Sometimes, suppliers cut corners or let stock sit too long. I’ve received shipments where crystal growth hinted at water contamination, or the liquid looked cloudier than expected. It pays to check reviews and audit reports when trying out an unfamiliar supplier. A conversation with the technical support team often tells more about quality standards than the product page ever could. In fact, some companies share public test reports; those always earned a little more trust on my end.
On the packaging front, waste presents a bigger headache than most expect, especially for smaller labs. Single-use glass or plastic ends up in the hazardous waste bin. Refill programs or larger containers could drop cost and environmental impact, though not every vendor wants to roll out those changes. Feedback channels stay important: asking for less packaging or more detailed purity info keeps pressure on suppliers and nudges the whole industry in a better direction. I've seen colleagues push for change, and every now and then, suppliers do listen.
Picking a source for 2-piperidin-2-ylethanol means more than scrolling through catalog numbers. Purity figures, packaging choices, and supplier transparency don’t just affect lab life—they steer the whole path from benchtop idea to finished product. Demand solid data, clear packaging protocols, and answers whenever questions surface. Backed by real experience and open dialogue, the best outcomes follow.
People who work with chemicals every day know this one truth: familiarity can breed carelessness at the bench, but certain compounds, like 2-Piperidin-2-Ylethanol, don’t forgive shortcuts. I remember walking into the lab as a first-year chemical tech, handed a simple plastic bottle with a name I couldn’t pronounce. The label had clear warnings, yet it was easy to gloss over the details in the rush to finish a task. So many accidents start there. Chemical safety is not about paranoia. It's about discipline and respect—habits that keep lab workers out of the emergency room, and businesses running smoothly.
Most chemicals don't belong on an open shelf. 2-Piperidin-2-Ylethanol falls in this camp. A cool, dry, and well-ventilated area gives this compound the best shelf life. Humidity invites clumping; heat introduces risks no researcher needs. Sealed glass containers, preferably amber, keep out both air and stray light, which can trigger unwanted changes. I've seen the results of a leak from a cracked jar — what should have been a straightforward day turned into hours of cleaning, paperwork, and lost trust. Label every container with the full chemical name, date received, and who opened it. This small step stops confusion during busy shifts.
Labs often hand out gloves and goggles at the door. Many ditch them after supervisors leave, claiming chemical resistance. Yet, 2-Piperidin-2-Ylethanol can irritate the skin and eyes—sometimes worse. Thin latex gloves, splash-proof goggles, and lab coats do more than tick a compliance box. On one project, a co-worker splashed a small amount, thinking it wouldn't matter for such a minor exposure. Instead, it caused a reaction that sent him to the nurse. Daily diligence pays back every shift.
Spills never greet you with a warning. Keep absorbent pillows and neutralizing agents ready. No one wants to scramble for supplies after a flask tips over. Clean minor spills with these materials first, then bag the waste and drop it in a secured chemical bin. For larger spills, evacuate and call in a professional cleanup crew. It’s a lesson you only need once. Waste disposal runs on clear labeling and proper separation—never pour it down the drain. Chemical mix-ups can generate fumes or reactions that hurt people and ruin equipment.
Several organizations offer safety training for handling specialty chemicals. In my experience, refresher courses actually keep safety knowledge from fading. Static posters and unread manuals gather dust, but hands-on practice sticks. There’s a huge difference between recognizing a hazard in a book and addressing one that crops up mid-experiment.
Double-check those standard operating procedures before starting work. Run through a quick mental checklist: fresh gloves, clear workspace, container tightly sealed, waste bins labeled, emergency contact numbers on the wall. This routine sounds simple, but it carves down risk every day. Chemical companies and research labs share one thing—everyone wants to clock out healthy.
As labs automate and regulations tighten, following strong safety habits grows even more important. Instead of shortcuts, build safety into every step. Those few extra minutes of prep and review keep people and workplaces productive, and everyone makes it home at the end of the shift.
| Names | |
| Preferred IUPAC name | 2-(Piperidin-2-yl)ethan-1-ol |
| Other names |
2-(2-Hydroxyethyl)piperidine 2-Piperidinylethanol 2-(2-Piperidinyl)ethanol |
| Pronunciation | /tuː paɪˈpɛrɪdɪn tuː ɪlˈθænɒl/ |
| Identifiers | |
| CAS Number | 2210-78-2 |
| 3D model (JSmol) | `/data/mod/jsmol/jmol.php?model=CC(O)N1CCCC1` |
| Beilstein Reference | 1713664 |
| ChEBI | CHEBI:19908 |
| ChEMBL | CHEMBL15901 |
| ChemSpider | 123435 |
| DrugBank | DB08218 |
| ECHA InfoCard | 03b7c5e0-1ad6-46a2-a12f-8d7e0e45b8fc |
| EC Number | 239-253-2 |
| Gmelin Reference | 1423116 |
| KEGG | C06360 |
| MeSH | D010901 |
| PubChem CID | 12323 |
| RTECS number | SJ5950000 |
| UNII | H8UM8578YT |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | DTXSID8063132 |
| Properties | |
| Chemical formula | C7H15NO |
| Molar mass | 143.23 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | characteristic |
| Density | 1.01 g/cm3 |
| Solubility in water | Soluble |
| log P | 0.0 |
| Vapor pressure | 0.0212 mmHg at 25°C |
| Acidity (pKa) | 10.02 |
| Basicity (pKb) | pKb = 2.84 |
| Magnetic susceptibility (χ) | -72.57·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.522 |
| Viscosity | 1.18 mPa·s |
| Dipole moment | 1.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –278.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4114.6 kJ/mol |
| Pharmacology | |
| ATC code | N07BB03 |
| Hazards | |
| GHS labelling | GHS02,GHS07 |
| Pictograms | C1CCCNC1CCO |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: "Keep container tightly closed. Wear protective gloves/protective clothing/eye protection/face protection. IF ON SKIN: Wash with plenty of soap and water. If skin irritation occurs: Get medical advice/attention. |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 97.4 °C |
| Autoignition temperature | Autoignition temperature: 270 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat) > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 2-Piperidin-2-Ylethanol: "LD50 (rat, oral) > 5000 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 50 mg/m³ |
| Related compounds | |
| Related compounds |
2-Piperidone Piperidine N-Methyl-2-piperidinemethanamine |