Chemistry grows from an old tradition of curiosity, and some molecules gather a story over many decades. 2-Piperazin-1-ylethanol isn’t a household name, but it has tracked through labs since the mid-twentieth century. In early years, piperazine derivatives drew attention for their potential in medicine and industry. Chemists noticed that introducing an ethanol group to the piperazine core gave rise to new reactivity. The first published syntheses appeared in specialized journals long before digital sharing, relying on simple glassware and fundamental organics knowledge. As research widened beyond Western Europe and North America, laboratories worldwide added their own tweaks and methods for building this compound, steadily laying a groundwork for broader access and study.
At its core, 2-piperazin-1-ylethanol comes from the coupling of piperazine and ethanolamine fragments, which folds the flexibility and burst of functionality chemists chase for more specialized builds. It’s a colorless to pale yellow liquid at room temperature, blending well with water and many organic solvents, which speaks volumes in any practical chemistry workflow. Its presence in industry reflects a blend of reactivity, moderate stability, and compatibility with complex synthesis. You won’t find it sold in corner shops but almost every catalog of chemical suppliers carries this intermediate. Known by alternative names like N-(2-Hydroxyethyl)piperazine or Piperazine Ethanol, it bridges research, manufacturing, and even regulatory filing with a certain reliability.
Measured up close, 2-piperazin-1-ylethanol stands out for being a crystalline solid with a moderate melting point, somewhere around 40°C. Its boiling point hovers near 300°C, so most reactions and storage run safely below any breakdown temperature. Its solubility in water and alcohols gives chemists elbow room to mix, react, and clean up without needing exotic equipment. The molecular formula, C6H14N2O, gives it both a reasonable size for manipulation and enough scope for functional group play. With a molecular weight of 130.19 g/mol, monitoring mass spectra or chromatograms becomes much easier. The molecule’s basicity remains tamed by the ethanol group, making it a dependable intermediate for reactions that don’t tolerate aggressive bases.
Strict batch labeling, purity assays, and quality control make this compound suitable for sensitive R&D and pilot production. Suppliers provide detailed safety data sheets (SDS) with accurate CAS number, batch identifiers, and expiration dates. Typical commercial grades arrive above 98% purity, which passes muster for most pharmaceutical synthesis and fine chemical industries. Chemical barcodes, detailed on the packaging, streamline lab inventory processes. Every package includes storage conditions and hazard classifications, compliant with regulations from REACH in Europe to OSHA standards in North America. Label transparency builds trust for scientists counting on batch-to-batch reproducibility and hassle-free regulatory inspection.
Chemists usually make 2-piperazin-1-ylethanol by reacting piperazine with ethylene oxide or 2-chloroethanol in a basic aqueous or alcoholic solution. This route generally avoids harsh reagents and allows temperature control, giving high yields if you keep the pH balanced and control the stoichiometry. Some labs prefer direct alkylation with excess ethanolamine, followed by purification through distillation or crystallization. Over time, changes in solvents, catalysts, and work-up steps have improved safety and efficiency. These processes benefit from straightforward monitoring, with TLC or HPLC tracking conversion and common spectroscopic tools like NMR confirming the product’s identity.
The true power of 2-piperazin-1-ylethanol lies in its twin reactive centers. The secondary amine in the piperazine ring invites acylation, sulfonation, or alkylation, letting researchers design molecules for diverse applications. The primary alcohol end reacts smoothly with acids, isocyanates, or through Mitsunobu conditions, opening access to esters, urethanes, or other custom groups. Medicinal chemists regularly harness both functionalities to fine-tune solubility or binding affinity in new drug candidates. This versatility has fueled its reputation as a valuable “building block” both in combinatorial synthesis and more directed organic projects. Extra steps sometimes even protect the ethanol group or convert it to a leaving group for ring-closure or chain extension reactions.
Depending on catalog or country, you might find 2-piperazin-1-ylethanol called N-(2-Hydroxyethyl)piperazine, 1-(2-Hydroxyethyl)piperazine, or Piperazine ethanol. CAS number 103-76-4 conveniently points buyers and regulators toward the exact compound, reducing confusion when similar molecules appear in literature. Some suppliers brand the product in proprietary ways, tying it to a line of amine intermediates or labeling for specific pharmaceutical routes. Recognizing these synonyms prevents costly ordering mistakes and research delays, which is crucial in fast-paced project settings where lab time means real money.
Handling 2-piperazin-1-ylethanol calls for the same discipline as most low molecular weight organics. Inhalation of vapor or skin exposure causes mild irritation, and safety data emphasize the need for eye protection and gloves. Labs usually set up local ventilation and spill containment even though the material doesn’t easily ignite or decompose. Its moderate toxicity profile makes it less hazardous than many reagents, yet regulatory compliance demands closed containers, correct waste disposal, and accurate labeling. Personnel receive regular safety training, and emergency protocols lean on well-rehearsed actions for chemical exposures. In industry, automated dosing and sealed blending keep both workers and the environment protected, showing lessons learned from decades of safe chemical stewardship.
Industrial uses center on its role as a versatile intermediate, enabling synthesis of active pharmaceutical ingredients, specialty dyes, and agrochemicals. In the pharmaceutical world, researchers often use it to build antiemetic, antihistamine, or anti-infective drugs, linking its structure to both solubility and metabolic stability tweaks. In the polymer sector, its alcohol and amine groups find use as chain extenders and cross-linking agents, fine-tuning hardness and flexibility in coatings or adhesives. Education labs sometimes adopt it for training on multi-step organic transformations, thanks to its manageable hazard level and the visually distinct conversion markers in test tubes and chromatography plates. This compound doesn’t just park in dusty chemical storage—it actively bridges research concepts and box-ready products.
People often forget the hidden journey that brings a chemical from academic paper to industrial scale. Investigators remain curious about new functionalizations of the molecule, exploring greener synthesis and ways to attach novel groups. Funding drives efforts into predictive modeling for better biological activity or reactivity, and machine learning algorithms crunch through structure-activity data faster than ever. Cross-disciplinary teams now look beyond classical chemistry, blending automated synthesis, AI-guided reaction route planning, and high-throughput screening. A focus on sustainability means labs look to reduce waste solvents and energy, drawing on real-world feedback from every failed flask and successful column.
Toxicity data for 2-piperazin-1-ylethanol show moderate risk profiles. Acute oral and dermal toxicity rates suggest careful but manageable use. Chronic exposure studies are rare, but regulatory filings and published research track its effects on animal models and eco-toxicity. Wastewater monitoring programs ensure disposal does not impact local waterways or trigger regulatory violations. Lab notes and incident records rarely show severe accidents when safety protocols are respected, but vigilance never fades. Ongoing research updates the global understanding, as even minor modifications or impurities demand close health and safety review.
Future interest in 2-piperazin-1-ylethanol depends on continued innovation across sectors. Rising attention to “greener” processes moves the field toward catalysts that lower energy needs or eliminate dangerous byproducts. AI and automation promise fresh pathways for both synthesis and application, reducing production costs and speeding up discovery. Regulations on chemical safety grow stricter, pushing for even tighter impurity limits and cleaner supply chains—both challenges and opportunities for suppliers and scientists alike. As new drugs and specialty materials demand ever-tighter synthetic control, the need for efficient and reliable intermediates only intensifies. The journey of this molecule reflects the broader story of chemistry: steady adaptation, creative problem-solving, and practical results built from old foundations and bold new tools.
2-Piperazin-1-ylethanol caught my attention years ago, messing around in the corner of a university lab, late after lectures. The chemical had a name that barely rolled off the tongue, but what it did in synthesis got people excited. This compound shows up most often in pharmaceutical factories and research spaces. It looks like a humble clear liquid, but its uses reach across medicine, industrial applications, and even agriculture.
Some chemicals land on a scientist’s workbench and just work—efficiently, reliably. 2-Piperazin-1-ylethanol often serves as a “building block” in drug manufacturing. Drug developers harness its structure to make active pharmaceutical ingredients. It’s particularly important for making medicines targeting neurological disorders. Picture antidepressants or antipsychotics; the synthesis route for these often features this ethanol-boosted piperazine. A lot of drugs with complicated backbone structures borrow a chunk from this molecule, helping them fit better into the biological receptors they’re made for.
Back in the nineties, stories floated around about chemists racing to create better antihistamines and anti-infectives. This compound helped doctors prescribe fewer sedating antihistamines, pushing progress in allergy medicine. It still plays that role, helping chemical labs craft new generations of treatments that reach millions. That’s real-world impact, backed by experience and hard data.
Labs often use 2-piperazin-1-ylethanol beyond drugs. Industrial chemists put it in as an intermediate to build specialty chemicals that wind up in products you see every day: cleaners, dyes, and sometimes agricultural products. The antifungal and antimicrobial properties of piperazine rings have been explored for making better crop protection agents. In fields and greenhouses, these substances stop fungus or bacteria before they can ruin harvests, supporting farmers who can’t risk a bad season. My conversations with a friend in agri-business highlighted the relief these innovations brought to small farm owners, who felt the pinch from pests every spring.
I’ve seen this compound tried out for water purification research too. In membrane manufacturing, certain piperazine derivatives can tune the properties of the filter, giving it greater resistance to foul water and longer usable life. Filtering clean water for villages short on resources—there’s no arguing with the practical importance of that kind of chemistry.
Like so many versatile chemicals, safety and handling form the core worries. Some users push for even greener chemistry, calling out the need for safer solvents and routes that avoid toxic byproducts. Research and industry are listening. Scientists look at new methods that cut down on waste and trim environmental footprints, all while pushing for higher yields. Students studying chemistry today want to know that what they use in the lab doesn’t undo the work of tomorrow’s environmentalists.
Regulatory agencies keep the pressure on, demanding transparent labeling and clear evidence of safety in consumer products. Researchers, policymakers, and industry tweak best practices together, knowing every small step toward greener production makes a dent in global chemical waste. For all its value to modern science and everyday products, 2-piperazin-1-ylethanol sits at the intersection of progress and responsibility. How we use it tomorrow depends on choices made in labs and boardrooms today, shaped by real-world needs and lessons learned the hard way.
Everyone who ever stepped into a chemistry classroom remembers the first time they saw a molecular diagram. Things looked chaotic, a mess of shapes and lines, but soon the logic came through. 2-Piperazin-1-ylethanol pulls me right back to those hard plastic molecular model kits. This compound brings together two pretty familiar elements if you’ve spent some time with organic molecules: a piperazine ring and an ethanol chain. It might sound complex, but at its heart, this molecule shows just how creative chemistry gets.
Let’s break it down. Piperazine forms that core ring – a six-membered structure, with nitrogen atoms where corners usually belong to carbon. Anyone who dabbled in medicinal chemistry or industrial synthesis probably ran into piperazine first in anti-parasitic drugs or chemical stabilizers. Its two nitrogen atoms open up all kinds of possibilities for attachment, binding, or modification in drug discovery or manufacturing.
The ethanol part of the molecule links up at the second position of the ring, almost like an arm reaching out. In plain terms, 2-Piperazin-1-ylethanol consists of that piperazine core with an ethanol group (CH2CH2OH) sticking off from one nitrogen. The real trick comes from this configuration—having the alcohol functional group off to the side brings solubility and reactivity that a plain piperazine ring can’t deliver.
In my time working in a university lab, we kept an eye on groups like these. The ethanol moiety made the molecule a lot more than just another ring system. It gave researchers leeway to build more complicated molecules—hooks for further chemical modifications. As straightforward as it sounds, the addition allows for attachment of widgets: fluorescent markers in bioassays, linkers for polymer chemistry, or even more involved pharmaceutical side chains.
Let’s not forget, innovations in chemistry often come not from wild, unfamiliar structures, but from subtle adjustments to existing skeletons. 2-Piperazin-1-ylethanol fits squarely in this camp. Tossing on an ethanol group turns a standard building block into something more versatile for drug synthesis and bioactive material development. For example, medicinal chemists use it as a scaffold in screening libraries and early-stage research, especially where the ability to dissolve in water and bond with other fragments gives a leg up.
Anyone designing molecules for targeted therapy, sensor design, or advanced polymers understands the drive to create flexible, modifiable platforms. 2-Piperazin-1-ylethanol provides just that. Its straightforward functionality makes it a good launching pad for synthesis without being as cumbersome as multi-ring or highly aromatic substances.
Like any tool in the lab, 2-Piperazin-1-ylethanol brings its own set of challenges. Sometimes, the secondary amines in piperazine become reactive at the wrong times. Sometimes that alcohol group gets stubborn, not attaching to what it’s supposed to. In my experience, the fix often lies in carefully choosing the order of reactions and protecting groups, and keeping conditions gentle. Using this compound well calls for a solid understanding of organic reactions and plenty of patience.
Researchers routinely look for better ways to tame these hurdles. Improved catalysts, smarter synthetic routes, and well-designed automation all play roles. Other chemists have begun exploring enzymatic pathways or mild, green chemistry solutions that keep those nitrogen and oxygen atoms in check.
2-Piperazin-1-ylethanol didn’t turn chemistry upside down, but it certainly gave people another lever to pull in research and industry. Its clear, modular design has turned it into a staple for those at the lab bench.
2-Piperazin-1-ylethanol isn’t a household name, but in chemical labs, folks bump into it more often than you might think. As a chemical, it works behind the scenes in pharmaceuticals and specialty chemicals, showing up wherever piperazine-based scaffoldings are needed. Everything with a piperazine ring deserves respect in the lab, and that’s a lesson learned by anybody who’s ever scrubbed a chemical burn from their hand because they took handling for granted.
Take it from the safety sheets and real-life mishaps: this chemical has a bite. You get it on your skin, it can cause irritation. Splash in the eye, you’ll remember it for days. Fumes aren’t as punchy as old-school solvents, yet confinement can still lead to headaches and coughs. Swallowing is an outright emergency. My first semester in chemical engineering didn’t mention colorful stories about spilled chemicals on pants, but after hours in the lab, watching skin turn red after the smallest drop, you start to respect the risk.
Not every chemical comes with a strong odor, and 2-piperazin-1-ylethanol can fool you there. The lack of an obvious warning scent doesn’t make it harmless. SDS documents underline that gloves and goggles aren’t optional. I remember working in a biotech startup where a misplaced flask turned into a week of treating a skin rash after skipping gloves in a rush. Chemicals only show mercy until they’re spilled.
Let’s talk numbers and studies. The European Chemicals Agency classifies it as posing risks on contact. Animal data points toward moderate oral and skin toxicity. It irritates eyes and skin, and some chronic studies hint at potential lung irritation if you’re careless with fumes over time. The CDC lumps it together with piperazine derivatives known for neurotoxic effects at high exposures. That sounds grim, but with the right habits, most labs avoid incidents entirely.
Every student and worker deserves to head home healthy after a day in the lab. For that to stay true, chemicals like this ask for full attention to detail. Even if it never turns up in the New England Journal of Medicine headlines, the risk of exposure is real. Ventilated hoods aren’t a luxury. Closed-toe shoes, gloves, and splash-proof goggles help you keep your skin and lungs safe. Nobody wins the productivity game by skipping steps for PPE.
Training plays a big role. The best labs I’ve worked in ran emergency drills and double-checked that everyone on shift could spot warning signs of exposure. I saw a culture shift when managers made room for short, open-ended discussions about mistakes and near misses, instead of waiting for disaster to teach the lesson for them.
Respecting chemistry isn’t about living in fear of every bottle in the store room; it's about habits. Ventilation, good labeling, immediate cleanup of spills, and clear lines of communication keep risk in check. If you see leaky containers or malfunctions in hood airflow, reporting and fixing them today beats hoping for luck tomorrow. That’s not abstract policy—it’s practical self-preservation.
With these steps, anybody handling 2-piperazin-1-ylethanol can do their job without rolling the dice on their well-being. In the end, safety comes down to knowledge, habit, and speaking up when something looks off.
A lot of lab professionals and buyers get stuck on figures when talking about chemicals, but not everyone stops to think about why purity makes a difference outside certificates and catalogs. For 2-Piperazin-1-ylethanol, purity isn’t just a sales point. It shows on the bench, in the final product, and sometimes in the hazards that show up when things aren’t what they claim. Whether someone’s working in pharmaceuticals, chemical synthesis, or biotech, low-grade material creates problems—reactions stall, yields drop, and safety takes a hit.
Most researchers expect at least 98% purity for 2-Piperazin-1-ylethanol. Top names in the chemical supply world, like Sigma-Aldrich and TCI, list this as their standard for general lab and pharma-grade supply. That small 2% can contain water, unknown synthesis byproducts, or related piperazine compounds. Some applications, especially early route scouting or quick pilot runs, can tolerate lower levels. For anything touching drug development or regulated synthesis, buyers will demand higher numbers—sometimes 99% or more, matched with full HPLC or GC trace.
My years benchside taught me how big an impact trace contaminants can have. During a scale-up project, we traced unexpected color changes to a lot of 2-Piperazin-1-ylethanol with unreported byproducts. That batch never made it to product launch. It wasted days and derailed the timeline. We thought nothing of a percent or two at first—the price was tempting. That “good deal” nearly doubled our costs in lost time and troubleshooting.
A spec sheet means nothing if the backup isn’t there. Labs today want the actual HPLC, NMR, or GC-MS printout for each lot—not just a blanket statement. Reputable suppliers post these results or deliver them with the shipment. The industry is moving toward more transparency, as buyers get burned by inconsistent supply from low-cost outfits. Some research groups, burned like we were, now run third-party verification—even on trusted brands. Passing the spec isn’t optional anymore; it’s checked, confirmed, and stored for audits.
Anyone who’s worked in custom synthesis or scale-up knows not every supplier hits the mark. For critical runs, always ask about batch-specific purity data and impurity breakdown. Push for a certificate of analysis (COA) listing assay, moisture, and heavy metals. If you’re unsure, lab-based HPLC is quick, and most university or contract labs can run a check for a reasonable fee. Also, avoid the cheapest vendor in the room—cost savings disappear quickly if you lose a batch or face regulatory pushback.
Over the years, I started keeping a running list of suppliers whose spec sheets matched reality and those who missed or fudged the numbers. Sharing info between labs helps everyone avoid repeat mistakes. If you come across persistent purity issues, report them through supply networks, as silent acceptance just lets problems grow.
The practical line for 2-Piperazin-1-ylethanol is drawn at not less than 98% purity for routine lab and pharma work. The real safeguard isn’t just the number, but evidence and a willingness to double-check before any large-scale or sensitive project. Better sourcing, transparent data, and more openness between buyers and suppliers keep projects on track and science moving forward.
Most people don’t encounter 2-Piperazin-1-Ylethanol in daily life, unless their days involve lab benches, drums, and safety goggles. This organic compound often pops up in pharmaceutical research, specialty coatings, or as part of chemical synthesis work. It’s colorless, may look unassuming, but it deserves respect from anyone handling it. Stories from friends in chemical supply have shown just how fast a misstep in storage turns into clean-up, wasted money, or in rare cases, health hazards.
Take heat and light off the table when storing this compound. Elevated temperatures, even in a casual stockroom, can speed up chemical reactions or break down the compound over time. From first-hand experience, a shelf near a window may feel convenient, but it often leads to higher internal temperatures. Too much heat, and the chemical can degrade—losing potency, forming byproducts, or at the worst, building up pressure inside its container.
So the best spot comes down to a stable, cool, and shaded location. A chemical refrigerator or a temperature-controlled cabinet keeps it around 20-25°C (68-77°F). Storage guidelines from industrial safety datasets reinforce the importance of avoiding both freezing and excessive warmth. While it’s tempting to squeeze one more bottle onto a shelf under a sink or by a radiator, this shortcut often leads to costly mistakes.
Moisture is another villain that rarely gets enough attention. Even a small leak, or opening the bottle too often in a humid room, can let water vapor in. This small misstep can change the chemical composition and potentially make the compound less useful. Service in a university chemical storeroom taught me to always check stoppers, avoid cracked seals, and keep desiccants close by. Keeping the compound tightly closed, with a dry environment, wards off slow but irreversible damage.
Crowding chemicals together because shelf space is tight isn’t rare. But stories of accidental mixing, vapor cross-contamination, and mystery residue in bottles are common. 2-Piperazin-1-Ylethanol asks for its own zone, away from acids, oxidizers, and much else. Chemical compatibility charts offered by regulatory authorities send a clear message: keeping incompatible chemicals separate isn’t just good practice, it’s about preventing reactions that nobody wants to deal with in person.
Once, during an inspection, an unlabeled bottle prompted hours of backtracking through purchase orders and emails. Missing or unclear labeling grows into a real risk. Professionally made labels with chemical name, date of opening, and hazard warnings cut down confusion. They help avoid accidents when work gets busy and people begin to forget what went where.
Routine inspections for leaks, discoloration, or compromised seals can seem dull, but they’ve caught problems early for every serious lab worker I’ve known. By keeping a log and regular schedule, teams behind the scenes keep everyone safer.
Even the best containers and protocols only go so far if nobody knows the rules. Short training sessions that highlight pictograms, storage maps, and emergency steps give even busy or inexperienced workers a solid foundation. My time as a safety trainer made me appreciate how storytelling—explaining not just what to do, but why—sticks much more than rote instructions ever did.
The bottom line: Storage of 2-Piperazin-1-Ylethanol doesn’t need endless checklists, but it does demand steady habits. Storing quietly in the right place, with the label facing out and the lid firmly shut, saves time and trouble for everyone down the line.
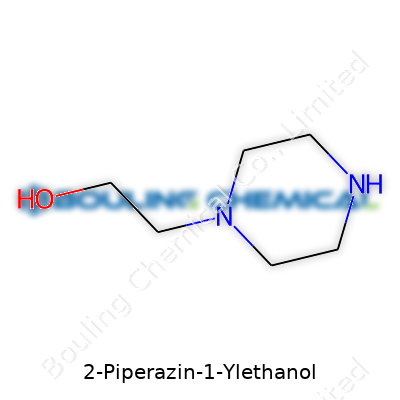
| Names | |
| Preferred IUPAC name | 2-(Piperazin-1-yl)ethan-1-ol |
| Other names |
1-(2-Hydroxyethyl)piperazine 2-(1-Piperazinyl)ethanol Piperazine-1-ethanol 2-Hydroxyethylpiperazine N-(2-Hydroxyethyl)piperazine |
| Pronunciation | /tuː paɪˌpɛr.əˈziːn wʌn ˈɪl ˈɛθ.ə.nɒl/ |
| Identifiers | |
| CAS Number | 103-76-4 |
| 3D model (JSmol) | `3DModel:JSMol("CCN1CCNCC1")` |
| Beilstein Reference | 157793 |
| ChEBI | CHEBI:34641 |
| ChEMBL | CHEMBL1506 |
| ChemSpider | 42771 |
| DrugBank | DB01174 |
| ECHA InfoCard | 100.043.431 |
| EC Number | 216-568-8 |
| Gmelin Reference | 79037 |
| KEGG | C06124 |
| MeSH | D010935 |
| PubChem CID | 71108 |
| RTECS number | SE7175000 |
| UNII | HR8600906V |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | DTXSID4020543 |
| Properties | |
| Chemical formula | C6H14N2O |
| Molar mass | 130.19 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Odorless |
| Density | 1.08 g/cm3 |
| Solubility in water | miscible |
| log P | -1.2 |
| Vapor pressure | 0.00024 mmHg at 25°C |
| Acidity (pKa) | 9.8 |
| Basicity (pKb) | 5.70 |
| Magnetic susceptibility (χ) | -41.4×10^-6 cm³/mol |
| Refractive index (nD) | 1.502 |
| Viscosity | 0.861 cP (25°C) |
| Dipole moment | 2.67 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 270.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -298.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −3706.8 kJ/mol |
| Pharmacology | |
| ATC code | N04BB02 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P362+P364 |
| NFPA 704 (fire diamond) | 1,2,0 |
| Flash point | 104 °C |
| Explosive limits | Explosive limits: 2.3–16% |
| Lethal dose or concentration | LD50 oral rat 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 2-Piperazin-1-Ylethanol: "1650 mg/kg (rat, oral) |
| NIOSH | TE7700000 |
| PEL (Permissible) | PEL: 15 mg/m3 |
| REL (Recommended) | 5 mg/m³ |
| Related compounds | |
| Related compounds |
N-Acylethanolamines Piperazine Ethanolamine 1-(2-Hydroxyethyl)piperazine N-(2-Hydroxyethyl)piperazine 1,4-Piperazinediol N-Methylpiperazine Morpholine |