2-Phenylpyrrolidine didn’t just pop into labs by chance. Chemists started digging into pyrrolidines back in the early 1900s, mostly because these ring-based molecules showed up in everything from natural products to potent pharmaceuticals. As synthetic chemistry moved ahead, adding a bulky phenyl group at the 2-position caught the interest of medicinal chemists. Over decades, they poked and prodded at this little scaffold, trying to coax out new drug candidates and better understand nitrogen-centered heterocycles. Before modern analytic tools, separation and purification were chores, and older synthesis routes spat out plenty of byproducts. It took careful detective work and repeated improvements in both reaction technique and purification to make 2-phenylpyrrolidine more than a lab curiosity. By the late 20th century, it found steady use in academic and industrial settings, often as a building block for bigger, flashier molecules.
2-Phenylpyrrolidine, a five-membered ring with nitrogen tucked inside and a benzene ring hanging off the second carbon, usually shows up as a clear or pale liquid, sometimes turning yellow if stored carelessly. Most scientists look for high purity, generally 97% and up for use in synthesis, though some specialty suppliers shoot for 99% or better. Small bottles, sealed tight and labeled with lots of warnings, sit on shelves in organic labs around the world. Pricing jumps around based on volume and grade, since the high-purity material takes more work and cleaner feedstocks. Some companies sell it alongside a handful of close cousins—substituted phenylpyrrolidines with fluorine, methyl, or other groups swapped onto the ring. These subtle changes can make a world of difference depending on the target chemistry.
Out of the bottle, 2-phenylpyrrolidine brings a mild, almost sweet smell that fades faster than some amines but lingers longer than some aldehydes. The boiling point settles around 238–240°C, which ranks it as a stable liquid under most reaction conditions. It pairs high solubility in common organic solvents like ether, dichloromethane, and ethanol, while barely mixing with water. The molecule resists shock and light, but strong acids or oxidizers chew it up quickly, transforming it into tars or breaking open the ring. With a molecular weight of about 161.24 g/mol, it works nicely in weighed reactions. The nitrogen pulls at protons and weak acids, yet doesn’t create the “fishy” stench typical of some amines. Purity checks rely on gas or liquid chromatography, with infrared and NMR spectra giving a clear signature for both the ring and the aromatic group.
Commercial bottles show hazard pictograms, since the pure compound irritates eyes and skin. Labels report the CAS number (771-68-2), assay (by GC or NMR), lot number, and sometimes generic names like “2-Phenylpyrrolidine” or “Pyrrolidine, 2-phenyl-”. Chemists expect neat product or solutions in solvents like toluene for easier handling. Data sheets warn against open flames, poorly ventilated spaces, and direct contact. Storage advice keeps it in amber glass under nitrogen or argon where possible, away from bases and strong oxidizers. Handling instructions encourage nitrile gloves, safety goggles, fume hoods, and quick clean-up of spills. If misused or dumped down the drain, local regulations kick in, treating it as hazardous or even as a precursor in controlled substance regulations in some regions.
In small labs, the go-to prep uses bromophenyl compounds and pyrrolidine with a base, often relying on transition metal catalysts like palladium. This Buchwald-Hartwig amination gives high yields but needs careful removal of metal residues. Other methods use reductive amination, where phenyl-substituted ketones react with ammonia surrogates under hydrogen gas with a metal catalyst. Each route balances scale, purity, cost, and leftover waste. Some hobbyists try old-school syntheses using sodium or potassium as a reducing agent, but these often lead to messy mixtures that take longer to clean up than to make. Commercial plants opt for continuous flow or carefully monitored batch reactors, focusing on quality and limiting byproducts. Analysts weigh in early, since a slight impurity profile can ripple through the value chain, especially if the material ends up in pharmaceutical R&D.
Once made, 2-phenylpyrrolidine rarely stays unchanged for long in chemist’s hands. Its nitrogen gets alkylated, acylated, or oxidized in countless experiments. Attaching bulky groups at the nitrogen turns the molecule into a chiral ligand for asymmetric catalysis. Substitutions at the phenyl position, like halogenation or nitro group addition, let researchers fine-tune both reactivity and biological activity. Using strong acids or peroxides opens or rearranges the ring, sparking whole new families of heterocyclic products. In my grad school lab, tinkering with the ring size sometimes led to home-brewed analogues for receptor assays—work best done under the supervision of a watchful principal investigator. Even now, simple tweaks to this molecule can produce entirely new patents, targeting everything from crop protection to enzyme inhibition.
Walk into any large chemical supplier’s database, and 2-phenylpyrrolidine crops up as “2-Phenylpyrrolidine,” “Pyrrolidine, 2-phenyl-,” “α-Phenylpyrrolidine,” and sometimes “1-Pyrroline, 2-phenyl-.” European catalogues might list it as “2-fenylpirrolidin.” I’ve seen it misfiled as “N-Phenylpyrrolidine,” which raises confusion since that label belongs to a differently substituted compound. Some pharma companies code it by research numbers or internal designations. Each name tells the same story of a five-membered nitrogen ring with a phenyl cap, but picking the right synonym avoids ordering headaches—especially in procurement systems juggling multiple languages or regulatory codes.
Contact with 2-phenylpyrrolidine stings skin and irritates airways, so most labs won’t open bottles outside a working fume hood. Gloves, face shields, and chemical aprons help keep splashes in check, especially in scale-up work. Emergency procedures focus on flushing exposed skin or eyes with water and using spill kits that bind organics. Labs treating this material as a precursor implement tight inventory controls and keep documentation straight. Disposal means collecting used material in solvent waste, with on-site incineration or licensed hazardous waste handlers taking the rest. Larger producers blend instrument controls, in-line monitors, and strict ventilation codes to minimize vapor exposure and leaks. These practices help both people and the environment avoid unnecessary risks, especially since similar compounds often show up on watch lists for their abuse potential.
Medicinal chemists like the 2-phenylpyrrolidine scaffold for making new drug candidates targeting brain receptors and antibacterial enzymes. The core ring shows up in opioid receptor ligands, anti-infectives, and central nervous system probes. Outside medicine, the molecule turns into ligands and chiral auxiliaries for metal-catalyzed syntheses. I’ve encountered it as a test substrate in methods development for chromatography and as an additive improving the selectivity in catalytic hydrogenation. Agroscientists and flavor chemists have dabbled in substituted derivatives, though food approval rarely follows due to safety limits. Analytical chemists use derivatives as standards, since the aromatic and basic sites improve detection sensitivity.
Academic labs continue to explore new derivatives, pushing to optimize both ring substitutions and N-modifications for better biological profiles. For every new batch, researchers publish analytic spectra, X-ray crystallography, or docking data matching the new compound to its potential targets. In pharma, combinatorial approaches have turned hundreds of these molecules into small libraries, hoping one sticks as a lead compound. Machine learning tools now help in predicting reactivity, toxicity, and likely off-target effects. Some work focuses on green chemistry routes, aiming to replace palladium or toxic bases with catalysts that are more sustainable, like nickel or iron. Armed with robust analytical tools, modern labs zip through projects that would have taken months in earlier decades, but new regulations constantly change what can be shipped, stored, or ordered internationally.
While 2-phenylpyrrolidine isn’t a staple of toxicology screens, its basic structure rings alarm bells for those investigating neurological effects. Rodent studies show moderate acute toxicity, though effects depend on the dose and route. Long-term exposure data remain sparse, especially for chronic low-level contact, but analogues impact neurotransmitter systems, leading to precautionary bans for misuse. Corrosive to eyes and mucous membranes, it also causes dizziness and nausea through inhalation. Modern safety data sheets flag not only immediate threats but also potential long-term reproductive and mutagenic risks, especially if stray nitrosamines lurk as byproducts. Some researchers now call for broader ecological studies, since run-off from industrial use may impact aquatic life, echoing concerns seen with other aromatic amines.
Looking ahead, chemists expect 2-phenylpyrrolidine to anchor yet more research into new CNS-active drugs and advanced ligands for asymmetric catalysis. Digital screening tools give a head start, predicting novel derivatives before the first flask ever leaves the shelf. Sustainability goals push green production processes, cleaner waste streams, and alternative reagents for both small-batch and industrial syntheses. Intellectual property fights already loom on the horizon as new analogues enter clinical trials. Safety and regulatory hurdles will only climb, especially as more jurisdictions crack down on compounds with psychoactive potential. With steady investment, young researchers will keep finding novel tweaks and clever uses for this flexible scaffold, cementing its place in chemical libraries and pharmaceutical toolkits.
Most folks outside a chemistry lab rarely cross paths with 2-Phenylpyrrolidine, yet this molecule carries serious weight in the world of pharmaceuticals and advanced research. At first glance, it looks like one of those names that only means something to chemists. Get behind the jargon, and the story gets a bit more interesting, especially when you start looking at how this compound shapes drug development and materials science.
In the lab, 2-Phenylpyrrolidine shows real potential as a building block for new medicines. The backbone of this compound pops up in several biologically active molecules, especially those with effects on the brain and nervous system. Scientists have explored its structure when tinkering with drugs aimed at treating depression, schizophrenia, or pain. Give chemists a framework like this, and they’ll tweak little bits here and there to test for activity against diseases that affect millions.
History teaches us that small changes in a molecule can lead to huge jumps in medical usefulness—or dangerous side effects. That’s why researchers gravitate toward cores like 2-Phenylpyrrolidine in their search for new medicines, especially ones that need to cross tricky barriers such as the blood-brain barrier. You don’t always get a blockbuster drug at the end, but every attempt teaches labs a little more about how the brain responds to different shapes and charges.
It’s tempting to see compounds like this as one-trick ponies tied only to drugs, but that doesn’t tell the whole story. Advanced materials science borrows lessons from molecules like 2-Phenylpyrrolidine. The unique ring structure lets chemists design materials with odd electrical properties or even act as ligands in metal catalysts. Some of these applications sound far off for regular folks, but they trickle down into everyday tech, including new polymers, clever coatings, or more robust batteries.
Any time labs get a molecule packed with both a nitrogen and an aromatic ring, they dream up countless experiments. Small tweaks in the lab can build up to big advances in the classroom, drug pipeline, or manufacturing plant. For example, the synthesis of 2-Phenylpyrrolidine lets students and professionals practice reactions like cyclization and asymmetric hydrogenation—reactions at the very heart of making new medicines cleaner, faster, and at lower cost.
Beyond offering training material, compounds such as this help move research away from the same old molecules. Pharmaceutical companies always need new chemical space to hunt for safe and effective drugs, and inventing sturdy intermediates gives those teams new ground to cover. If decades searching for better antidepressants or painkillers have shown anything, it’s that the tiniest structural difference can push a drug from useless to life-saving.
Not every molecule lives up to its promise, and there are hurdles. Some versions of 2-Phenylpyrrolidine prove tough to make or purify at scale, so labs always chase smoother and greener synthetic routes. Limiting hazardous byproducts and cutting down waste means a lot to anyone watching the long-term environmental footprint of pharma and chemical manufacturing.
Put simply, though the average person may never hear its name, 2-Phenylpyrrolidine underpins a surprising amount of effort in moving medicine and technology forward. Sometimes, the unsung chemicals in the background matter most, shaping better health outcomes and smarter tools for the future. As the search for new cures and cleaner chemistry rolls on, the place for this compound seems pretty secure.
Sometimes, chemistry feels like a collection of intimidating names, but each one opens a door to something real. Take 2-Phenylpyrrolidine as an example. At first glance, the name throws a curveball, but its structure turns out to be more approachable than expected.
Picture a five-membered ring—one of the building blocks for a lot of medicines and natural products. In this case, the core is a pyrrolidine, which carries four carbon atoms and a little nitrogen, all connected in a tight ring. Attached to the second carbon on that ring, a phenyl group extends out—six carbon atoms linked in a familiar benzene hexagon, a bit like a small chain bracelet hooked to a loop.
Putting it down simply, the chemical structure of 2-Phenylpyrrolidine can be written as C10H13N. The arrangement looks like this: start with the pyrrolidine, and then take a phenyl ring (C6H5-) and tack it onto the second carbon (position 2) of that ring. Chemists sketch this out with the nitrogen at the top of the ring, numbering the carbons clockwise, so the phenyl group always claims the second spot. In diagram terms, the structure shows the phenyl sticking out from carbon 2, while the nitrogen waits at the head of the table.
Learning the pattern of 2-Phenylpyrrolidine reaches beyond academic curiosity. For anyone who works with pharmaceutical chemistry, these small tweaks in molecular structure drive medicinal power or downfall. In some labs, adding or removing just that phenyl group transforms a basic ring into an active ingredient for medication. A famous example comes from research into central nervous system drugs. Scientists take the pyrrolidine foundation, swap out different groups at the second carbon, and suddenly, new effects on the brain appear.
Chemists love 2-Phenylpyrrolidine because it ties into a class known for impacting neurotransmitters. It pops up in studies about dopamine and stimulants, connected with the kind of compounds explored in both medicine and, less gloriously, the black market. Sometimes this leads to heated debates over controlling its use, since simple changes can spark new designer drugs. That poses a real challenge: tweaking one chemical group sidesteps law, yet the effect on health can spiral out of control.
Regulation and safety checks don’t just need new laws, they need chemists and policymakers finding common ground. Tracking these changes means keeping pace with new compounds and understanding where a tweak might turn therapeutic into harmful. Training more people in identifying alterations at the molecular level boosts both drug safety and detection of illicit variants.
Education can’t stop at the lab workbench. Getting the word out to doctors, pharmacists, and even regular folks means fewer surprises down the line. Simple resources explaining the impact of swapping a phenyl group or shifting a nitrogen atom offer a buffer against unintended consequences. For me, learning about ring structures and attachments gave new respect to the chemistry behind everyday medicines—and a new sense of urgency about the role each group plays in what happens inside our bodies.
Peeling back these layers pushes chemistry from paper exercises into real life. No molecule floats free from consequence, and understanding their structures helps everyone keep control over what’s possible, both good and bad.
Ask anyone who’s ordered lab supplies: purity can twist your brain into knots. With 2-Phenylpyrrolidine, this gets even trickier. Some folks swear by technical grade, others demand the “99+%” badge that costs more than a decent dinner. People throw around terms like HPLC or GC, and you’re left wondering if your next batch of chemistry will blow up or succeed.
People assume the highest purity is always the goal—real life rarely lines up so cleanly. In a university lab, for basic synthesis or classroom demos, no one pulls out the magnifying glass. Often, 95% purity checks the box and fits the budget. Walk across the hall to the pharmaceutical side, though, that’s a different tune. Pharmaceuticals, peptides, research involving sensitive reactions—all those demand that extra decimal.
Purity isn’t just a marketing gimmick, either: impurities can trip up chemical reactions, wreck results, and sometimes throw a wrench in projects worth thousands of dollars. I once witnessed an entire batch of new chemical targets fail because no one cared what sort of “trace salts” lurked in the bottle. That lesson burned a hole in the group’s budget, and nobody shrugged it off afterward.
Suppliers push out 2-Phenylpyrrolidine in more flavors than people realize. Technical, lab, and analytical grades—each one slots into a different job, with price climbing as you crank up the purity.
Technical grade usually serves basic, large-scale syntheses where you expect to scrub or purify things down the line. Lab grade sits in the middle. Analytical or “ultra pure” options might land in the mail with certificates, melting point data, maybe even a chromatogram. It’s not for show—some research stakes its reputation on the tiniest difference.
People shelling out for top-shelf purity sometimes pay more than the chemistry benefits. Many reactions tolerate a pinch of impurity without blinking. In some industries (perfume, basic bulk production), technical grade products keep prices under control and don’t drag quality underwater.
Food for thought: chasing purity often means extra chemical processing, more energy, higher waste, and higher cost. It’s an environmental hit that only makes sense if your end goal needs it. The smarter approach means matching your purchase to your real need—not the marketing hype. In my own work, I've seen smaller labs stretched thin, waste time chasing high spec materials they never needed.
Before buying, check what you truly require for your project and what your budget can take. Batch certificates, SDS sheets, and supplier reputation all help clear the fog. Even a quick check-in with someone down the hall—instead of relying on vendor promises—can save both money and headaches. Smaller labs sometimes band together to buy higher grade materials in bulk if they all need them. For the rest of us, settling for a little less shine often gets the job done just fine.
At first glance, 2-Phenylpyrrolidine looks like just another colorless liquid in a row of flasks. Chemistry labs have their fair share of such bottles, but anyone who’s accidentally cracked open a container with no safety plan knows one truth: every substance carries its own risks. This compound is no paragon of stability. Mishandling brings real danger not only to the person holding the pipette but also to anyone sharing the same workspace.
2-Phenylpyrrolidine doesn’t catch fire on a whim, but its vapor won’t do your lungs any favors. Prolonged exposure, even at low levels, can cause irritation. Splash on your skin and you’ll know it. Pour it into the wrong container or leave it in the light, and degradation becomes a headache. Over the years, I’ve seen colleagues clean up spills with nothing but worried looks and a stack of paper towels, leading to days of headaches and extra work. Manufacturer safety data sheets aren’t just paper drills — they exist because these risks are real and can interrupt research or even force a lab shutdown.
Personal experience shapes most habits. The best way I found to store 2-Phenylpyrrolidine always starts with a tightly sealed container, made of glass or high-quality HDPE. The label needs to be bold, with clear writing showing the full chemical name, concentration, and date opened. I don’t trust simple masking tape and pens that fade with solvent splashes — invest in good labeling.
The storage spot makes a big difference. Keep this compound away from moisture and direct sunlight. A cool, dry cabinet with a lock works best, preferably a well-ventilated flammables cabinet set off from the busiest parts of the workspace. People make mistakes grabbing bottles on crowded shelves, so keep this one separate from acids and oxidizers. Do not store above eye level. Dropping a glass bottle from height always leads to panic.
Safety goggles, gloves, and a lab coat are not optional. This is non-negotiable. Wiping down containers before shelving them isn’t just about tidiness—it prevents future residue buildup and cross-contamination. If a spill happens, the right spill kit should sit within arm’s reach, stocked with absorbent material that actually neutralizes rather than just soaks. I’ve lost count of labs where these kits go missing or sit empty.
Do not store more than you expect to use over a few weeks. Smaller quantities mean less risk, both in terms of exposure and waste. If you find expired or leftover material, arrange for pick-up via your facility’s hazardous waste channel promptly. Old containers gather dust and do nothing but tempt shortcuts.
Complacency sneaks in fast, especially after hundreds of hours working around unfamiliar names. Those shortcuts with labeling, ignoring drip stains, or parking bottles in the wrong place—these slowly build toward disaster. In my own work, a little discipline up front saves hours of unwelcome cleanup or, worse yet, an emergency call. The rules aren’t just red tape—they’re lessons written in past mistakes.
People who work with chemicals soon learn that not every vial or bottle should be approached the same way. 2-Phenylpyrrolidine may not have the headline name that some industrial chemicals do, but it deserves respect. This compound tends to show up in specialized organic syntheses, and its chemical profile looks a bit friendlier than some of its cousins. That deceptive calm can fool a technician into thinking the risks are lower, but overlooked hazards still lurk — especially if you’re the one opening the bottle.
Take one step into any laboratory, and you’ll see the gloves, goggles, and fume hoods. Old safety posters always showed dramatic scenes: a dropped beaker, an explosion, a fireball. Chemical safety more often hides in the slow things: vapors, skin contact, or a splash to the eye. 2-Phenylpyrrolidine isn’t especially volatile, but breathing its vapors over time or absorbing it through the skin still brings trouble. Those in academic labs or processing plants see their share of quick hand washes and near-misses with splashes. Continuous exposure, even to less dramatic chemicals, can build up — and often the body doesn't warn right away.
Reminding folks to wear nitrile gloves and lab coats can sound like nagging, but it's advice written in experience. Anyone who’s skipped gloves “just for a quick weigh” has a story that usually doesn’t end with a clean pair of hands. Chemicals like 2-Phenylpyrrolidine don’t always stain or burn on contact, but they love small cuts or thin skin. More than one chemist has found out that stinging sensation in the nail beds means the gloves weren’t good enough. That’s a lesson that sticks.
Goggles seem uncomfortable at times, but the quickest accidents often send liquids straight toward the eyes. The eye’s surface practically pulls in anything unfortunate enough to land there. Chemists I’ve worked with carry mental lists of colleagues who’ve made trips to the eyewash in their careers. It’s rarely because someone planned to get hurt—it’s because the routine got too comfortable.
Storage matters a lot more than folks might think. 2-Phenylpyrrolidine prefers cool, dry places with good ventilation, but even on the storage shelf, temperature swings or humidity can mess with purity or prompt reactions with other compounds. Label everything—more than one bottle of mystery liquid has forced a lab group to waste hours deciding whether to treat it as toxic waste.
Accidents can and do happen. Spills should never wait until after a coffee break. My own experience taught me to keep absorbent pads and chemical spill kits close by. An ounce of preparation means a spill doesn’t spread, ruining equipment or endangering more people. Having clear instructions nearby about what to do, and actually reviewing them, makes all the difference when seconds count.
A safe workplace doesn’t come from a list of rules, but the habits those rules build. If you’re using 2-Phenylpyrrolidine, set up every bit of personal protection, even if the job “will only take a minute.” Routines like handwashing, labeling, double-checking seals, and clearing clutter go from small chores to true lifelines. Mistakes don’t only cost time, materials, or comfort — sometimes they cost much more. The right habits, repeated day after day, turn safety into second nature.
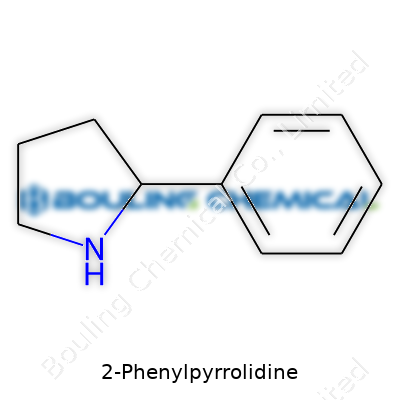
| Names | |
| Preferred IUPAC name | 2-Phenylpyrrolidine |
| Other names |
2-Phenylpyrrolidine 2-Phenyl-1-pyrrolidine 2-Phenylpyrrolidin |
| Pronunciation | /tuː-ˈfiːnɪl-pɪˈrɒlɪdiːn/ |
| Identifiers | |
| CAS Number | 1003-98-1 |
| 3D model (JSmol) | `/pdb/2phy/jmol/` |
| Beilstein Reference | 3539416 |
| ChEBI | CHEBI:132799 |
| ChEMBL | CHEMBL170220 |
| ChemSpider | 168141 |
| DrugBank | DB08798 |
| ECHA InfoCard | 100.123.398 |
| EC Number | EC 254-645-7 |
| Gmelin Reference | 085502 |
| KEGG | C15606 |
| MeSH | D064370 |
| PubChem CID | 136725 |
| RTECS number | SE8354000 |
| UNII | V9Y85I5ZOY |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C10H13N |
| Molar mass | 147.22 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | aromatic |
| Density | 1.049 g/mL at 25 °C |
| Solubility in water | Slightly soluble |
| log P | 1.95 |
| Vapor pressure | 0.02 mmHg (25°C) |
| Acidity (pKa) | pKa ≈ 11.2 |
| Basicity (pKb) | 3.36 |
| Magnetic susceptibility (χ) | -75.6·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.574 |
| Viscosity | 1.009 cP (20 °C) |
| Dipole moment | 2.04 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 380.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3688.7 kJ/mol |
| Pharmacology | |
| ATC code | N04BC12 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P271, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | Flash point: 111°C |
| LD50 (median dose) | LD50 (median dose): Oral, rat: >2000 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 1.0 |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
2-Phenylpyrrole 3-Phenylpyrrolidine N-Phenylpyrrolidine Pyrrolidine 1-Phenylpiperidine |