2-Phenylpyrrole came onto the scene thanks to the curiosity-driven tinkering that powered organic chemistry through the 20th century. Early chemists, always on the hunt for new materials, started searching for derivatives of pyrrole that packed more punch in terms of reactivity and utility. Once they figured out how to slip a phenyl ring onto the pyrrole backbone, the molecule stood out. Researchers quickly realized this wasn’t just a curiosity; the structure offered real opportunities in making compounds for everything from OLEDs to pharma intermediates. Over the years, as analytical tools sharpened and synthesis routes got cleaner, more and more labs adopted 2-Phenylpyrrole as a staple starting point or building block. What started as a bench-top curiosity ended up as a regular feature in research catalogs and chemical supply houses.
2-Phenylpyrrole doesn’t just offer a mouthful of a name—it gives chemists a handle for elaborate synthetic work. Sitting at the intersection of aromaticity and electron-rich reactivity, this compound is favored for its unique electronic profile. The molecule’s popularity spans from dye chemistry and fluorescent probe development to industrial-scale production of pharmaceuticals and fungicides. Folks in the lab prize it for that balance: enough reactivity to be versatile, but also stable enough for storage and handling. 2-Phenylpyrrole usually gets shipped as a colorless to pale yellow oily liquid, sometimes as a low-melting solid in cooler climates, and its purity often touches 99% when sold for research or manufacturing.
Taking a closer look at the bottle, 2-Phenylpyrrole brings a molecular weight of 169.22 g/mol, with a chemical formula of C10H9N. Its melting point falls around 24°C (75°F), just above room temperature, and it boils between 290°C and 295°C. The compound dissolves well in common laboratory solvents like ethanol, acetone, and diethyl ether. It gives off a faint, characteristic aromatic odor—nothing overpowering, but it’s there. Chemically, it behaves as expected for a pyrrole: electron-rich, eager to donate in electrophilic aromatic substitution, reluctant to play with strong acids or bases except under forced conditions.
On most product labels, purity takes top billing—lab suppliers usually market 2-Phenylpyrrole with purity above 98%. Impurities like biphenyl or unreacted pyrrole sometimes pop up, flagged through GC-MS or HPLC analysis. Labels also mark batch number, CAS number (notably 636-73-7), and recommended storage temperature (often just “keep sealed, cool, and dry”). Regulatory warnings like GHS labels note that the compound may cause skin or eye irritation, so gloves, goggles, and sometimes fume hoods are recommended, especially in volumes above a few grams. Long shelf life can be expected unless the container stays open for weeks at a time.
If you've spent time at a laboratory bench, you know that synthetic methods evolve fast. Initial manufacturing relied on cross-coupling methods like Ullmann reaction between bromopyrrole and phenyl halides, but yields were never anything to brag about. With advances in catalysis, modern shops prefer Suzuki-Miyaura coupling between pyrrole boronic acids and phenyl halides, riding the efficiency of palladium-based catalysts. This cleaner approach skips over most byproducts, speeding up post-reaction workup and improving yields north of 80%. For anyone running this on a multi-gram-to-kilogram scale, sticking to Suzuki-Miyaura makes cleanup and downstream purification manageable.
2-Phenylpyrrole becomes the chemical equivalent of a Swiss Army knife in the right hands. That spare nitrogen on the ring lets chemists dial in a wide range of substitutions. N-alkylation and N-acylation reactions shape all kinds of new molecules for medicinal chemistry workflows. Electrophilic substitution hits the pyrrole ring, delivering halogenated, sulfonated, or acylated derivatives. For dye and OLED makers, functionalizing the phenyl ring through Friedel-Crafts or cross-coupling opens up a floodgate of new possibilities for adjusting color or charge mobility. Redox chemists enjoy tinkering with the electronic structure too, often by pushing electrons onto the ring system and monitoring how fluorescence shifts with each change.
This compound picks up plenty of alternative labels depending on who’s selling it or citing it. Some catalogs call it 2-Phenyl-1H-pyrrole. Others shorten it to alpha-Phenylpyrrole or employ the IUPAC name for clarity. You might spot other synonyms like o-Phenylpyrrole or 1H-Pyrrole, 2-phenyl-. Most suppliers stick to the CAS number 636-73-7 as a touchstone, since naming conventions swing between academic and industrial preferences. Checking against the molecular structure image is always smart when ordering to avoid mixing it up with structurally similar isomers.
Handling 2-Phenylpyrrole means taking common laboratory precautions seriously. While the compound isn’t wildly toxic at the typical concentrations used in research and industry, it does irritate the skin, eyes, and respiratory tract if you let it get out of hand. Think of the standard toolkit: gloves, safety glasses, lab coat, and a properly working fume hood. In my experience, accidental splashes won’t send you to the ER, but ungloved handling or poor ventilation during evaporation leaves you with that distinctive pyrrole itch or a mild headache. Proper labeling, secondary containment, and odor minimization help keep things smooth. Waste containing this compound gets collected for hazardous disposal according to local regs—no shortcuts.
The reach of 2-Phenylpyrrole cuts across more fields than most expect. Organic electronics folks rely on it to craft new materials for OLEDs and light-harvesting devices, where tuning the emission or charge properties matters more than ever. Pharmaceutically, it anchors routes to antifungal agents like fludioxonil, a crop protection active ingredient. Dye chemists draw on its electron flow for new classes of fluorescent or near-infrared markers. It even pops up in the search for new antipsychotics and pain meds, where the core gets tweaked for target-specific binding. Anyone developing new material with a need for both aromatic and heterocyclic character takes another look at this molecule, especially for proof-of-concept syntheses.
Having a flexible molecule like 2-Phenylpyrrole opens the door for more than a handful of projects. Labs across Europe, China, and the US push the limits of this chemical for everything from solar concentrators to fungicide resistance management. Structure-activity relationship (SAR) work thrives on derivatization, since swapping side groups leads to measurable shifts in potency or material properties. My time running undergraduate synthesis labs taught me that students quickly grow to appreciate how subtle changes to the phenyl or pyrrole rings deliver big shifts in observable color or NMR signature. Major companies and academic groups keep working up “library” sets of substituted derivatives, hoping for the next big hit in either sensors, pharmaceuticals, or electronic device layers.
Safety testing for 2-Phenylpyrrole doesn’t spark as many headlines as some newer synthetic molecules, but data still stacks up over time. Acute toxicity remains low in animal models, with LD50 numbers in the gram-per-kilogram range—far less dangerous than most lab solvents or reagents. Still, chronic exposure studies flag mild hepatotoxicity at very high doses, and the compound gets tagged as an irritant, especially for skin and eyes. Studies on environmental persistence suggest moderate risk, mostly due to breakdown into simpler pyrrole-based metabolites. For agricultural or pharmaceutical applications, regulators keep a close eye on breakdown products and possible cumulative effects in soil, water, or animal systems. That’s driven a push toward new derivatives that offer rapid degradation or easier metabolic handling in living systems.
The story for 2-Phenylpyrrole doesn’t look set to end anytime soon. More industries hunting for advanced materials, better diagnostics, or greener agrochemicals keep coming back to this structure, tinkering with side groups and core modifications in search of something better. Computational models point to even richer optical and electronic properties if the right substituents get dialed in on the phenyl or pyrrole rings. Environmental concerns drive research into biodegradable or metabolically safe derivatives, and electronics demand purer, more controllable forms. The next chapter for this molecule likely involves more specialized applications—OLED displays, targeted pharmaceuticals, or next-generation sensing platforms—using both classic wet chemistry and novel green methods to get the job done.
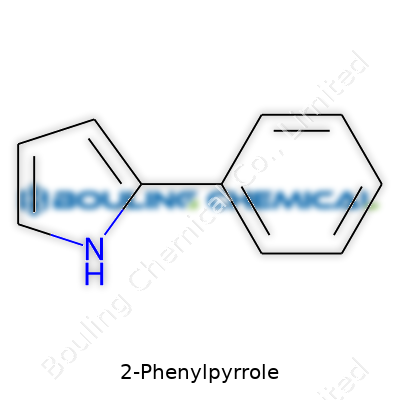
2-Phenylpyrrole does not sound like the start of a practical story, but plenty of things turn on small pieces like this one. This compound brings together a pyrrole ring, a nitrogen-containing building block, with a phenyl sidekick, forming a simple-looking molecule with a surprising punch.
Organic light-emitting diodes (OLEDs) draw power from the unique properties of compounds like 2-Phenylpyrrole. Screen tech has always chased better colors and thinner displays. With 2-Phenylpyrrole, it’s the efficient transport of electrons and its influence on the light’s spectrum that earns it a spot in the recipe for brighter phone screens and TVs. My own phone’s color depth and crisp lines owe a little to molecules optimized for this kind of work—the difference is pretty obvious when you compare cheap old screens to new ones packed with such chemistry.
During my undergrad years, I watched 2-Phenylpyrrole play a role far from the spotlight. Students and researchers keep reaching for it in the lab while making more complicated organic molecules. It acts as a piece in puzzles that mimic natural dyes, antiviral agents, or anti-inflammatory drugs. Things always feel more real when instead of pictures in a textbook, you stand in that smelling, humming room measuring out white powder, then running reaction after reaction, checking which route gets the cleanest yield. In those cramped labs, even a tiny bit of a compound like this opens up routes to new stories for medicine and material science. Published papers from recent years spotlight how chemists start with this simple structure and tweak it to pull out new biological tricks—often hunting molecules that can target cancer cells or nasty bacteria.
Farmers face tough odds with every growing season. Crops get hit by diseases that wreck yields and threaten food supplies. Fungicides built from 2-Phenylpyrrole derivatives have formed a line of defense against molds and root diseases. Unlike some “classic” compounds that lose their punch after a few seasons, this chemical group found ways to knock out fungal resistance—meaning the sprays stay useful longer. What stands out here is how farmers saw real effects, with some fields reporting rescued harvests and less blight, thanks to sprays traced back to this molecule. It helps keep food on the shelves and prices steadier. The hard part remains: balancing persistence in the soil and potential environmental side effects, which calls for more research and tighter regulation.
Nothing comes for free in chemistry. 2-Phenylpyrrole offers help but asks tough questions, too. As screens get brighter and farms get more efficient, we see heat building up in e-waste and worries over run-off in fields. Safer protocols and recycling plans should move to the front, so the chemical gains don’t come with surprise losses down the line. Research budgets need attention, pushing green chemistry so that new versions reduce side effects and lower hazards. Teaching students why these molecules matter—not just how to make them—builds a generation that thinks hard before scaling up in the real world.
Step into any research lab and you’ll find someone with a markered flask, muttering about a molecule. Among them, 2-Phenylpyrrole gets its share of attention. Its molecular formula stands as C10H9N—a short stack of letters and numbers, carrying quite a bit of punch. I remember early in my chemistry training, a professor drew the structure on the board and described it as “a pyrrole ring hooked to a benzene.” This pairing pulls double duty: it keeps things relatively stable, but the nitrogen in the five-membered pyrrole adds just enough unpredictability to make it interesting.
Picture a pyrrole ring, which is a five-sided shape made from four carbons and one nitrogen. Attach a benzene ring (six carbons in a perfect hexagon, with alternating double bonds—we called these “aromatic” in class, which just means the electrons move around a lot) to the second position on that pyrrole. That spot sits right next to the nitrogen. So, if the nitrogen gets numbered as spot number 1, then attach benzene to spot number 2.
The actual shape matters, not just for textbooks but for real-world reactions. Molecules like 2-Phenylpyrrole don’t just float in space—they interact, bind, and stack. Stick them in the right context, and suddenly you’ve got the backbone for new drugs, novel materials, or even light-emitting devices. The rigid rings and that central nitrogen set the stage for interesting chemistry, from hydrogen bonding to electron flow.
You’ll find people chasing after 2-Phenylpyrrole when designing things like organic light-emitting diodes. Its conjugated system—the way those double bonds talk to each other—helps carry electrical charge, making it a good candidate for use in display screens or specialized sensors. It’s not as famous as caffeine or aspirin, but several studies push it into the spotlight for anti-inflammatory research and materials science. If you work on synthetic chemistry, building up molecules like this comes up all the time; it’s almost like laying bricks for a new wall, except each brick connects in a fascinating way.
Learning to draw this molecule by hand taught me to respect the discipline of organic chemistry. There’s a logic to how the rings meet, why the hydrogen atoms anchor themselves on certain points, and how the lone pair on nitrogen can reach across the structure, tugging at bonds. If you ever catch a chemist tracing hexagons and pentagons on a napkin, chances are they’re working through problems just like this. Real insight into these molecules opens doors, whether you’re testing drug candidates or developing next-gen electronics.
Getting 2-Phenylpyrrole pure isn’t always straightforward. Aromatic rings encourage all sorts of side reactions during synthesis. Labs work to improve yields, control impurities, and keep costs down. Some researchers aim to make the process greener—swapping harsh solvents for water or ethanol, cutting waste, and finding cleaner catalysts. Chemistry never stands still. As methods improve, more complex derivatives start showing up in patents and papers. Tinkering with “where the rings join” leads to molecules with surprising properties—improved light emission, new biological activity, or easier handling.
Ultimately, understanding the formula and structure gives us more than just trivia for a pop quiz. It unlocks a toolbox for scientists, offering pathways to smarter medicines and smarter materials. The basics matter, even for a seemingly simple molecule like 2-Phenylpyrrole. Pay close attention to those rings and bonds; in those connections lies a world of possibility.
I’ve seen plenty of labs and storerooms over the years, some pristine, others a patchwork of forgotten bottles and sticky floors. One thing always catches my attention: how people treat the bottles with weird, unfamiliar names. 2-Phenylpyrrole, for many, falls into that category. This compound pops up in organic syntheses and research labs, and it draws its share of worry with the unknowns around toxicity and stability. It’s easy to grow numb to warnings if you pour over Safety Data Sheets all day, but ignoring the basics just tests your luck.
Let’s start with storage. Cool, dry, well-ventilated — those are not just nice-to-haves. 2-Phenylpyrrole should go in a spot away from sunlight and heat because chemical degradation gets real in a warm, sunny window. Humidity can mess with the purity too, so skip the shelf above a sink or under a window. Strong odors and unwanted reactions make good reasons to keep the lid tight and bottles upright, far from oxidizers and acids. I like storing such materials in chemical-resistant containers, with clear, bold labels that stick well and can’t be peeled off by sweaty fingers or the odd solvent spill. It’s less about following a regulation and more about knowing what's there on a busy day.
Early in my career, I watched someone skip gloves because “it wasn’t anything dangerous.” That thinking gets people into trouble. Even if toxic data for 2-Phenylpyrrole seems limited, skin contact and inhalation add up over time, and no one wants a mystery rash. Latex and nitrile gloves serve well, and a pair of safety goggles makes a world of difference — not just for splashes, but for annoying powders that float up exactly when you least expect it. I double up on protection when unsure about dusts or fumes, especially if the lab hood’s airflow has taken the day off. Basic lab coats keep splashes off clothing, so you don’t track residue around like breadcrumbs.
Any spill, big or small, gets people tense, and that’s justified. I always have absorbent pads handy, plus bake soda for neutralizing spills, and heavy-duty bags or containers to scoop up any solid matter quickly. 2-Phenylpyrrole needs to go in a hazardous waste stream — never down the drain or tossed in the regular trash. Local waste management rules exist for a reason, even if they feel like paperwork-heavy hoops.
Colleagues lean on each other's habits. I have learned more from watching careful veterans than from dry training slides. Locking up storage areas, double-checking container labels, and logging chemicals keep messes from turning into health scares. Lapses in safety culture show up fast in any group, so I speak up if I spot open bottles or broken labels. Safe work is a team effort, not just a solo checklist item.
Chemicals like 2-Phenylpyrrole won’t advertise their own risks. Respecting their potential with real-world caution just makes daily life smoother. It doesn’t take an industrial accident to prove a point — habits shape health and keep disasters out of the news. Most mistakes are avoidable by setting up your space for safety and holding yourself to those habits, day after day.
Anyone who's ever spent time around a chemistry lab knows that the tiniest contamination can throw whole experiments off track. The same goes for the world beyond the bench—pharmaceuticals, advanced materials, and agrochemicals all put a premium on purity. With 2-Phenylpyrrole, this chemical’s grade draws a clear line between success and frustration.
In most labs, 2-Phenylpyrrole turns up at purity levels pushing 98% or even higher, sometimes specifically labeled "analytical" or "reagent" grade. For work that demands pinpoint precision, anything under 98% feels risky. A colleague of mine once tried a synthesis using a 95% version, and the yield dropped so sharply we thought the method was flawed. Only swapping in a 99% batch brought things back in line. It’s a classic case where “almost pure” isn’t close enough.
Research shows high-purity batches often contain less than 0.5% total impurities, based on common quality control methods like NMR and HPLC analysis. These numbers aren’t just technical posturing—if you’re chasing a new pharmaceutical compound, you want the cleanest starting point. Impurities sometimes trigger unwanted byproducts, or worse, sneak into a final product that ends up in a medicine cabinet or in food chain studies. The stakes run high.
Packaging often sets the tone for how chemicals get handled. Besides the technical aspects, the options reflect a real need for safety and practicality. Glass bottles in 25-gram or 100-gram sizes tend to show up most in catalogues—these are favorites for academic labs or folks testing out small reactions. Glass offers solid protection from air, and students quickly learn that a well-sealed glass bottle keeps their reagents dry and uncontaminated for longer. My group usually ordered the 100-gram size for a semester’s worth of research, and that felt just right for both shelf life and budget.
Polyethylene containers start to appear with larger amounts, like 500 grams or a kilo. These have the edge when shipping long distances or in rougher environments—plastic survives drops that shatter glass. Once, during a conference in Mumbai, a supplier told me their plastic jugs saved them hundreds in shipping claims thanks to less breakage. Some products come vacuum-sealed, especially with grades purer than 99%, as exposure to moisture or air can quickly degrade quality. Those in the field know how even a brief whiff of humidity can leave a clumpy mess in an open drum.
Bulk packaging, such as 25-kilo fiber drums with plastic liners, is available from industrial suppliers. At this scale, safety jumps even higher in importance—regulations require clear labeling, spill-proof sealing, sometimes even tamper-evidence. In my time advising a startup scaling up pesticide precursors, I saw what happens when bulk drums are left exposed or improperly sealed. Besides losing material, entire supply chains get delayed due to failed audits or impure product batches.
For anyone worried about safety or loss of quality, small steps go a long way. Training people to transfer chemicals with dry scoops, not leaving bottles open, and recording every time a drum is accessed can stop most contamination. Switching to smaller, sealed bottles rather than continually dipping into a single vat also helps avoid repeated exposure to air. Labs on a tight budget often pool requests with nearby colleagues, ensuring chemicals arrive in the right size, are used quickly, and don’t sit forgotten.
Manufacturers see the demands changing as regulations tighten and green chemistry comes to the forefront. More companies now publish not just purity numbers but also detailed impurity profiles, so buyers know exactly what’s inside. For those in the field, these details can make sourcing less of a guessing game and more of an informed decision—saving time, money, and sometimes, whole research projects.
2-Phenylpyrrole pops up in research more often than most folks realize—think organic chemistry labs, and even as a stepping stone in creating fungicides. I remember sweating over reaction equations in college, my hands reeking of solvents and a dozen unpronounceable compounds. I never gave much thought to the paperwork attached to the bottles. Only later did I realize how much rides on those Hazard labels. With 2-Phenylpyrrole, you really can’t ignore the fine print.
2-Phenylpyrrole isn’t just some inert chemical sitting quietly on a shelf. Contact with skin or eyes can irritate, sometimes pretty badly. Inhalation can cause headaches, dizziness, and even trouble breathing if you’re in a poorly-ventilated room. Years of keeping chemicals in cramped rooms with fans humming in the background taught me to respect what’s floating in the air, even when it’s nearly invisible. I watched classmates cough after a dropped bottle spread an invisible cloud throughout the room. A quick scrub at the eyewash station beats a trip to urgent care, but it’s best to avoid that dance entirely.
There’s a bigger picture too—some studies suggest long-term exposure to pyrrole compounds could impact liver health, though there’s no clear consensus on 2-Phenylpyrrole specifically. I’ve seen older techs in labs who handle things with a distant wariness—they’ve heard the stories, seen colleagues pulled off the floor for mysterious health checks. Uncertainty shouldn’t mean carelessness.
Across the United States, the Environmental Protection Agency (EPA) lists chemicals like 2-Phenylpyrrole in databases, tagging them for manufacturers to track. The EU tosses this compound onto candidate lists, which means you spot it in the REACH databases too. Over the years, watching paperwork multiply for every bottle and batch taught me two things: requirements may sound tedious, but they exist for a reason, especially when chemicals show up in products moving across borders.
Companies must supply Safety Data Sheets (SDS) for anything shipped or stored, listing risks and how to handle them. Facilities label storage areas, restrict access, and keep spill kits handy. Inspectors drop by to check that all’s in order. I once worked somewhere that failed a surprise visit—locked cabinets left half-open, and a spill response plan no one remembered. We spent the next week retraining, hands cramped from rewriting logs and safety checklists. It drove home the value of those rules, even if they chase you from shelf to shelf.
Good ventilation ends up as the unsung hero in places that mix or use pyrrole compounds. Fans don’t get much love, but working all day with volatile chemicals teaches you to appreciate a reliable air filter. Gloves, goggles, and lab coats become second nature. In one place, I watched a new hire ignore the gloves for supposedly “benign” organics—he picked up a rash that stuck for days.
Old habits die hard, especially around routine. But routine leads to risk, not safety. Even if 2-Phenylpyrrole doesn’t carry explosive risk or cause cancer on contact, taking shortcuts leads to trouble. The right habit—checking labels, using extractor hoods, reading up on new guidelines—beats recklessness every time.
Real safety sticks when people care enough to push for better. An open dialogue, up-to-date training, and honest inspection matter as much as any EPA label. Stories from my time in busy research labs still echo back: a little vigilance and shared knowledge combine to guard against what can’t be seen. That’s where regulation matters—not in red tape, but in how people walk out whole at the end of a shift.
| Names | |
| Preferred IUPAC name | 1-Phenyl-1H-pyrrole |
| Other names |
2-Phenyl-1H-pyrrole o-Phenylpyrrole 2-Phenyl pyrrole |
| Pronunciation | /tuː-ˈfiːnɪl-pɪˈroʊl/ |
| Identifiers | |
| CAS Number | 2306-90-5 |
| Beilstein Reference | 110639 |
| ChEBI | CHEBI:34082 |
| ChEMBL | CHEMBL125691 |
| ChemSpider | 20588806 |
| DrugBank | DB08345 |
| ECHA InfoCard | 100.023.872 |
| EC Number | EC 620-519-1 |
| Gmelin Reference | 85882 |
| KEGG | C06595 |
| MeSH | D019276 |
| PubChem CID | 70410 |
| RTECS number | UJ4375000 |
| UNII | N3X3JL51GZ |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C10H9N |
| Molar mass | 169.22 g/mol |
| Appearance | Light yellow crystalline powder |
| Odor | aromatic |
| Density | 1.105 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.9 |
| Vapor pressure | 1.39E-02 mmHg at 25°C |
| Acidity (pKa) | 23.2 |
| Basicity (pKb) | 12.11 |
| Magnetic susceptibility (χ) | -52.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.634 |
| Viscosity | 1.26 mPa·s (25 °C) |
| Dipole moment | 2.21 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 246.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 87.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3237 kJ/mol |
| Pharmacology | |
| ATC code | N03AX04 |
| Hazards | |
| Main hazards | Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P305+P351+P338, P321, P332+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | Flash point: 113°C |
| Autoignition temperature | 651°C |
| Lethal dose or concentration | LD50 (Oral, Rat): 2500 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 640 mg/kg |
| NIOSH | SN9800000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/kg |
| Related compounds | |
| Related compounds |
Pyrrole N-Phenylpyrrole 2-(2-Thienyl)pyrrole Indole 2-Bromopyrrole |