Looking back at the path chemistry has taken, 2-Phenylimidazole has always reminded me how a simple tweak in molecular structure sparks new directions. It first showed up in literature in the early half of the twentieth century, springing from the curiosity around heterocyclic compounds and their mysterious activities inside living systems. As organic synthesis matured, so did methods for building imidazoles with all sorts of aromatic additions. By the 1960s and 1970s, labs were already recognizing this compound not just as a building block but also as a potential lead in medicinal chemistry. Over the last few decades, the depth of research into imidazole derivatives has only increased, especially with today’s hunger for pharmaceutical innovation and advanced polymer chemistry. Reading those old research papers, you sense the excitement each time someone found a new way to make the ring or attach a different phenyl group—which often changed reactivity in ways nobody expected. These historical notes aren’t just trivia; they set the stage for why this molecule turns up wherever reactivity, bioactivity, and synthetic utility matter.
2-Phenylimidazole stands out as a small, aromatic heterocycle, part of a larger family of imidazole compounds. Size-wise, it weighs in at about 144.17 g/mol—compact, but with a punch from its aromatic core and the nitrogen pairs in the ring. Most folks in chemical supply know it comes as a clean, white crystalline powder, easy to spot on a benchtop among bottles of less distinctive chemicals. Its scent isn’t sharp or overwhelming, but one that rings familiar to anyone who’s spent enough hours in an organic lab. As a molecule, it’s not just a spectator—it plays roles in crosslinking resin systems, promoting curing reactions, and sometimes lending its character to new classes of pharmacological agents. Whether in a large facility making materials at scale, or a small research group probing how slight molecular changes affect activity, 2-Phenylimidazole rarely lies unused for long.
Talking properties brings everything practical into view. 2-Phenylimidazole melts around 145–147°C—a handy range that slots well with polymer processing and organic synthesis. Solubility varies with solvent, but most polar organics like DMSO or ethanol dissolve it with little complaint. In water, it manages some solubility, though nowhere near the levels you see from polar molecules without those bulky rings. Its structure puts electron-donating phenyl next to two nitrogen atoms, making it an interesting proton acceptor and lending it a slight basicity—pKa hovers near 7, so it won't upset most reactions needing stable, gentle bases. Stability at room temperature avoids headaches during storage, and its resistance to slow oxidation or hydrolysis saves more samples than many realize. Because its physical form rarely changes after long storage, warehouses and labs alike prize it for shelf life.
Industry standards for 2-Phenylimidazole aren’t rolled out just to satisfy paperwork—quality really matters. Manufacturers usually supply a minimum assay of 98% purity, ensuring few side products pop up during downstream reactions. Impurity profiles typically get documented, especially residual solvents, water content, and ash values. Every bottle gets labeled with the CAS number (670-96-2), warning signs about possible irritation, storage advice (dry, cool space), and recommended disposal pathways. Labels also spell out whether batches pass frequently requested tests for heavy metals, which gives buyers confidence their material won’t introduce unknown variables. In practice, labs hold onto certificates of analysis, not out of habit, but because a trusted label can be the difference in troubleshooting a tough reaction or failed product run. I've run into situations where a subtle quality dip led to inconsistent yields, underscoring that specifications aren't something to skip over or treat like a mere regulatory hurdle.
Making 2-Phenylimidazole occupies a central space in organic synthesis, especially during heterocycle construction. The typical process starts with benzil, ammonia, and glyoxal in a condensation that closes the imidazole ring, followed by isolation under acidic or basic conditions. For years, the classic Radziszewski synthesis served as the gold standard. Chemists use both batch and continuous processes, with solvent selection swinging between methanol, ethanol, or sometimes acetic acid, fine-tuned for scalability or environmental impact. Yields usually shoot upwards of 70-85%, especially when reaction temperatures and times are dialed in through careful monitoring. Some newer protocols pivot towards solvent-free or green chemistry routes, using catalysts like zeolites or ionic liquids to reduce waste. Seeing these tweaks in person gives you a clear sense: synthetic chemistry stays dynamic, driven not only by efficiency but by rising environmental concerns and safety demands.
In the lab, 2-Phenylimidazole plays well both as a reactant and as a substrate for further modification. The most striking thing is its rich reactivity—electrophilic substitution at the ring, N-alkylation, or even oxidative couplings with aryl halides deliver new derivatives without much fuss. Under basic conditions, alkyl halides add to the nitrogen site, providing handles for larger molecule construction. In pharmaceutical R&D, acylation or sulfonation sprinkles in bioactivity or shifts physical properties, producing compounds that researchers screen for all sorts of disease targets. The aromatic ring tolerates mild bromination or nitration, expanding the landscape for structure-activity relationship studies. As with most heterocycles, reactivity demands respect: improper conditions foster unwanted tars or resinous byproducts, so process chemists keep the book of reaction troubleshooting within arm’s reach. The versatility of 2-Phenylimidazole never comes without knowing your solvents, catalysts, and purification tricks.
Too often, confusion springs from a crowded shelf of nearly identical chemicals. 2-Phenylimidazole doesn’t hide behind many aliases, but it still picks up synonyms in catalogs and journals: Benzimidazole, C6H5C3H3N2, 2-(Phenyl)imidazole, or simply PI. The absence of a single naming convention can trip up an inexperienced chemist, especially with older literature opting for varied structures like "alpha-Phenylimidazole" or "1,3-diazole, 2-phenyl-." Commercial producers sometimes tag it as an "imidazole accelerator" in the curing resin industry, confusing if you’re matching product specs without reading the fine print. Reading both historical and modern labeling underscores how crucial it is to double-check everything—from CAS registry numbers to IUPAC nomenclature—before buying or synthesizing this compound for critical applications.
Safety measures start at the bench and work their way up through every level of handling. 2-Phenylimidazole doesn’t qualify as the most hazardous compound in a chemist’s arsenal, but direct skin or eye contact irritates, and inhalation of dust invites similar complaints. Working with gloves, goggles, and dust masks turns into second nature after a week or two in any production facility. Its relatively low toxicity makes it less worrisome than many amines or aldehydes, but chronic exposure—especially as an airborne particulate—raises flags for long-term respiratory health. Spills often resolve with basic sweeping and dilution, but disposal always follows local regulation, treating all aromatic amines as potentially harmful. Most suppliers issue SDS (Safety Data Sheets) that spell out procedures for accidental exposure, fire hazards, and compatibility, and because of this, thorough safety training isn’t just for compliance but makes real impact on accident prevention. Over the years, I’ve seen how good operational standards actually reduce downtime, and equipment lasts longer where dust control and spill protocols exist.
2-Phenylimidazole holds a broad range of uses—not limited to one industry or style of chemistry. In epoxy systems, its role as a curing accelerator cannot be overemphasized; it pops up in adhesives, electrical insulation, and advanced composites. Resin chemists reach for it to boost cure rates, lower process temperatures, or tweak the mechanical properties of finished products. Pharmaceutical researchers put it under the microscope as a scaffold for antifungal, antimicrobial, and anticancer candidates. Cropscience leverages its derivatives for selective fungicidal action, especially in places where resistance to older agents rises quickly. Analytical labs sometimes use it as a ligand or complexing agent, especially in methods involving transition metals. The sheer range of its influence—from industrial processing to life science innovation—reminds me that small molecules, properly harnessed, carry enormous impact across disparate fields. Where new challenges surface, especially in material science or medical discovery, this compound seems ready for another act.
Academic and industrial R&D gives 2-Phenylimidazole fresh relevance every year. Chemists constantly develop new modifications to unlock antiviral, antimicrobial, or anti-inflammatory properties. Pharmaceutical companies target the heterocyclic core for lead discovery, trying to tune bioactivity while managing toxicity and pharmacokinetics. Polymer science stands as another hotbed: ongoing work refines the additive’s activity in toughening resins or improving flame resistance. These efforts don’t just populate journals or patents—they drive concrete improvements in product reliability and patient outcomes. Trends in green synthesis push for alternative routes that minimize hazardous byproducts, such as enzyme-mediated ring closures or photochemical additions. Close industry-academic collaboration means findings rapidly transition from bench to pilot scale—something I’ve witnessed in real-time collaboration projects. The pace of innovation, especially combining computational modeling with empirical tweaking, continues to open new combinations and applications every year.
Toxicology work on 2-Phenylimidazole didn’t take off until fairly recently, as its industrial use and pharmaceutical promise ramped up. Acute toxicity values (LD50 in rats) hover well above those of more hazardous amines, though caution persists over its chronic effects. Metabolic fate in mammals indicates modest absorption and efficient excretion, though some hepatotoxic potential surfaced in high-dose rodent studies. Current data don’t show widespread carcinogenicity or mutagenicity, yet regulatory bodies request ongoing studies into long-term occupational exposure, especially for manufacturing workers. Environmental toxicity seems moderate, with rapid breakdown in aerobic soils but persistence in waterlogged environments causing some concern in aquatic ecosystems. For anyone handling the compound in scale-up, these results reinforce the push toward closed systems, fume control, and careful wastewater management. The toxicology record may seem mild for now, but as usage expands, so will the mandates for more rigorous oversight and ongoing monitoring of workplace exposure limits.
The future for 2-Phenylimidazole looks robust, marked by both industrial reliability and scientific curiosity. Demand for its use in rapid-curing epoxy systems, specialty adhesives, and functional coatings shows no signs of fading—if anything, the push for higher-performance, lower-tox materials keeps it relevant. Pharmaceutical research persists; as the search for new heterocyclic scaffolds continues, chemists will likely push the core ring into new therapeutic territory. Environmental and toxicological research will play catch-up, especially around new modifications and waste management. Advances in green chemistry—think solventless synthesis, recyclable catalysts, and in-line purification—will probably shape production methods, lowering the burden of hazardous waste. What I notice most is how its versatility puts it on the shortlist for up-and-coming research projects across both material science and biomedical spheres. Standing at the crossroads of innovation and regulation, 2-Phenylimidazole won’t be fading into obscurity soon; it keeps earning its spot as a cornerstone of both practical manufacturing and exploratory science.
Talk to any chemist about a raw compound, and "purity" jumps out right away. It isn’t just a number on a label: it's the key to what that chemical can actually do. Step into a lab or scroll through a chemical distributor’s website, and you’ll notice 2-Phenylimidazole showing off purity percentages like 98%, sometimes even higher. That number looks reassuring. But what sits behind it? For anyone who spends time handling fine chemicals, 98% purity doesn’t always feel as clear-cut as it sounds.
That stray 2%—the part that isn’t 2-Phenylimidazole—can cause serious headaches or, with the right luck, be harmless. Years in an organic synthesis lab taught me what these impurities do. One time, I thought I found a bargain deal on a 98% labelled bottle for a new project. Project deadlines pressed, I went ahead. Testing melted points and running TLC plates showed strange smears and odd shifts. The impurity, invisible at first glance, messed with my product yield. Even in analytics, those unwanted extras can end up masking or mimicking important signals, pushing analysis off track.
Big manufacturers know this pain. Purity isn't only about chemical reactions. For industries making electronic parts or pharmaceutical ingredients, it’s about avoiding entire batches’ worth of ruined product, or passing strict regulatory gates. Trace metals or leftover solvents might sound minor, until they chop points off device lifespans or meet a drug regulator’s "fail" stamp.
Any company selling compounds like 2-Phenylimidazole usually relies on classic techniques: thin layer chromatography, melting point checks, and sometimes high-performance liquid chromatography. The chemistry department’s patience with titrations and overnight drying cycles pays off. Purity, in these cases, is more trust than science, unless someone shows you a full HPLC profile or spectroscopy readout. That’s why labs or buyers often double-check—nobody wants to repeat a week’s worth of work because someone cut a corner.
For many buyers, the challenge comes down to cost. Those extra few percentage points of purity can drive price up, and not everyone needs the highest purity for routine benchwork. I’ve seen professors order lower-grade batches to save budget, planning to purify the compound themselves. Students end up with glassware full of strange crystals—part chemistry lesson, part money-saving project.
Yet once you’ve struggled through hours of clean-up, the value of starting with something closer to "chemical perfection" bites differently. Development teams and researchers pay for that reassurance. Pharmaceutical work almost always demands purities of 99% and above. Sometimes, regulatory inspectors go beyond surface certificates, pulling samples to check for the tiniest traces of toxic byproducts.
A big part of finding better purity sits with suppliers. Strict protocols for storage and handling matter, since 2-Phenylimidazole can soak up moisture or yellow in bad conditions. Improved packaging, batch testing, and transparent reporting push the whole market forward. In my experience, partnerships with those suppliers who invest in solid quality controls make life in the lab easier.
For anyone starting a project, asking about the real meaning behind "98%" or testing it in-house before running big experiments saves stress. Sometimes, lab groups share tips on the best vendors or smartest ways to purify rougher material on their own. Scientists talk about purity not as a brag, but as the difference between progress and wasted days.
2-Phenylimidazole may not ring a bell for folks who don’t work in a lab, but it's behind many things we bump into daily. It's easy to overlook building block chemicals like this, but their impact quietly shapes a lot of products we count on. I’ve come across 2-Phenylimidazole on labels during my college chemistry days, and it's wild to look back later and see just how much ground it covers, especially in industries you wouldn’t expect.
In adhesives and coatings, cures matter a lot. 2-Phenylimidazole steps in as a curing agent—making sure epoxies set fast and hang tight in tough spots. I remember helping a friend fix some floor tiles, and the epoxy glue worked its magic, holding things in place day in and day out. The secret sauce often traces back to compounds like this, delivering that quick, hard set when you need repairs or protection.
Take a close look at your phone case or a home appliance, and you’re staring at another end-product of epoxies that use 2-Phenylimidazole. Printed circuit boards would be a pain to make without this chemical. The circuit bits have to stick to the board so electronics can survive knocks, heat, and moisture. Adding this imidazole to the epoxy means gadgets last longer and don’t quit working after a rough drop.
This compound doesn’t show up on medicine bottles, but drug companies use it to build bigger molecules. It helps form the backbone in some antifungal and antiviral drugs. Chemists chase after such intermediates because they handle tricky chemical steps that lead to reliable medicine. Every time I reach for something to soothe a sore throat, it’s easy to forget about the chain of reactions kicking off with simple molecules like 2-Phenylimidazole.
Ever wonder how your dyed t-shirt keeps its color wash after wash? Dye and pigment makers lean on stable chemical structures to stop fading. 2-Phenylimidazole steps in again, helping lock the pigment in so the color stands up to sunlight and soap. There’s something odd but kind of cool about seeing science at work every time you do laundry.
Pipes, tanks, and even bridges battle rust every day. Industry experts add 2-Phenylimidazole to paints and coatings to stand between steel and the elements. This means less patching and replacing of expensive gear. Coming from a family of mechanics, I can tell you rust is no joke, and every bit that helps fight it means fewer breakdowns and lower bills.
Plenty of research explores using 2-Phenylimidazole in greener, safer material science. The more we unlock about its chemistry, the fewer toxic ingredients we need for things like coatings and drugs. Industry can keep building on this with smarter manufacturing and policies that encourage less waste. As awareness grows, companies might share more about their supply chains so we can follow the path from raw material to finished thing in our hands.
Straightforward science, real-world impact—the story of 2-Phenylimidazole keeps growing as technology moves forward.
We run across chemicals every day, most of them tucked away on product labels or buried deep in research reports. One of them is 2-Phenylimidazole, a name that doesn’t sound familiar unless you’ve studied chemistry or worked in a lab. It doesn’t pop up in ordinary conversation, but its story highlights how chemistry feeds quietly into modern life. The molecular weight—this basic number—tells us a lot about how it moves through the world.
2-Phenylimidazole offers a molecular weight of 144.17 grams per mole. For chemists, this is the sort of simple fact that shapes everything from how much you dissolve in a beaker to how substances interact in a reaction. I remember back in college, carefully measuring out fine white powders on a steel balance; it took patience, but not knowing the right weight meant all the work could go down the drain. Too much or too little, and the reaction wouldn’t line up. Mistakes cost time, money, and sometimes safety.
Every educator drills this point: chemical reactions demand precision. 2-Phenylimidazole’s molecular weight turns up when someone weighs out a reagent for an experiment, working out how molecules pair up, or even when scaling a reaction for manufacturing. Industrial chemists especially pay attention, since buying or using the wrong quantities creates expensive waste or unsafe processes. Safety data sheets list these numbers plainly, and for good reason—this weight links to everything from dust inhalation limits to storage rules.
These numbers don’t exist just for bored students with calculators. A small example: pharmaceutical teams exploring new drug compounds often look for molecules in a certain weight range. Large molecules can’t move through the body easily. 2-Phenylimidazole, sitting in a sweet spot, gives scientists the freedom to experiment without fretting about size barriers. A compact molecular weight means the compound clears one of the earliest hurdles for testing as a drug candidate or building block.
Molecular weights also shape the way chemicals travel and break down in the environment. Think about what happens if a spill hits the ground—lighter molecules evaporate or move with water, while heavier ones often settle or resist breakdown. Environmental engineers look at these details when deciding if a compound will stick around in soil or disappear after a summer rain.
Mistaking the weight of 2-Phenylimidazole isn’t some academic slip-up. Over the years I’ve seen labs forced to repeat full batches because a single decimal got ignored. More than that, getting it wrong can put workers at risk if they handle or dispose of too much of a chemical substance. A little extra care early in the process—double-checking weights, reading the label twice—avoids accidents and wasted effort. Beyond the four walls of the lab, these habits become part of any system that values safety, reliability, and respect for the real-life impact of science.
Chemistry won’t become less complicated anytime soon. If anything, the growing push for greener, safer materials means scientists lean more than ever on basic details—like molecular weight. Industry standards for labeling, smarter balances for measurement, and open training all drive the right habits. Sharing this knowledge, and repeating it in classrooms and on the job, means fewer people have to learn lessons the hard way.
I once worked in a cramped university lab, where unusual smells and chemical containers lined sagging shelves. We often took shortcuts, storing nearly everything in reused glass jars or faded plastic bottles. One afternoon, a leaking cap on a bottle of unidentified powder set off the fire alarm. That day, I realized something simple: storing chemicals right isn’t just about compliance, it’s about everyone getting home safe.
2-Phenylimidazole shows up in many research and industrial settings. You’ll find it in synthesis labs, materials science work, and even in polymer manufacturing. Lab folks often get too casual with things that look like just another white powder. But a compound like this can degrade if you store it wrong—moisture sneaks in, heat kicks off reactions, or vapors build up. Poor storage leads to ruined experiments or, in tough cases, nasty health risks.
From my own hands-on time, every shelf fight and cleanup drill taught me that moisture and air are always trying to get inside. 2-Phenylimidazole slowly absorbs water from the atmosphere. Once it gets clumpy, your calculations are thrown off—yields drop and byproducts increase. You don’t want to see sticky lumps where there should be a dry, free-flowing powder.
Keep it in tightly sealed bottles, preferably glass with PTFE-lined caps. That’s not being fussy; some plastics will leach or react, especially with solvents or under light. If you’ve ever opened a drawer to find a warped plastic jar and a fine sprinkling of unknown dust, you know the frustration. Dry glass bottles cut that headache off early.
Heat is sneaky. I’ve seen chemicals lose strength just from sitting near a sunny window or on a crowded radiator shelf. Most imidazole derivatives stay solid and safe at room temperature, as long as the temperature doesn’t swing too wildly. Basements and windowless storerooms, away from heat and sunlight, work best. Labeled cabinet spaces in a cool, dry room help ensure nobody grabs the wrong bottle or lets something overheat. Direct exposure to bright light doesn’t do 2-Phenylimidazole any favors; over time, light can encourage gradual decomposition, leaving you with degraded, less reliable material.
One problem that creeps up in multi-purpose labs comes from keeping incompatible chemicals too close together. Once, someone stored organics right next to nitric acid. The shelf rusted, labels were lost, and nobody trusted those powders again. With 2-Phenylimidazole, keeping it at arm’s length from strong oxidizers, acids, or bases makes a difference. Separate shelves, clear labeling, and good housekeeping stop accidents before they start.
Storing chemicals right might sound tedious, but it’s less work than cleaning up leaks or tossing out spoiled material. Stock up on high-quality, labeled glass containers. Place silica gel packets in storage cabinets to keep things extra dry—swap them out every few weeks, especially in humid environments. Keep up-to-date inventory sheets, so you always know what’s on hand and catch any suspicious changes early. Small habits like these lower risks and save budgets.
Attention to detail—in storage as much as in experiments—builds trust, keeps people healthy, and protects investments. Each bottle handled with care makes every step in the lab a little safer and every result a little more reliable.
Every manufacturer asks a similar question at some point: “How much of this can I get, how fast, and how consistent will it be?” I’ve lived through days scrambling after obscure reagents. 2-Phenylimidazole is not exactly a household word. It does pull its weight in specialty chemical production – catalysts, corrosion inhibitors, pharmaceutical intermediates, and certain advanced polymers lean on compounds like this. Suddenly, scarcity or delays in sourcing can raise costs, halt R&D, or even threaten product launches.
On paper, 2-Phenylimidazole looks straightforward enough. Several lab-chemical suppliers offer grams or hundreds of grams in well-sealed bottles. Those options dry up quickly for anyone who needs tens or even hundreds of kilos. A quick email to catalog chemical distributors rarely brings much joy above a few kilos; the price climbs and the lead time stretches. Big manufacturers don’t keep excess sitting on a shelf.
A few bulk producers do synthesize 2-Phenylimidazole on order. Chinese and Indian chemical plants have shown capacity for multi-ton production runs. That route often means minimum order quantities in the hundreds of kilos, up-front payment, and testing shipments before betting the farm on a supplier. In my time, direct conversations – not catalog clicks – uncovered the true availability. Sometimes a phone call does what no email request form manages.
Sourcing this compound in bulk always raises an anxiously practical question: can you trust the quality over time? With 2-Phenylimidazole, impurities from the synthesis pathway can fuel headaches in pharmaceuticals or specialty applications. Smaller suppliers sometimes stick to lower-purity grades to keep prices tempting, but final products may slip out of spec or underperform.
Back in the lab, we sent samples for independent analysis rather than gambling with an unknown batch. This step weeds out the poorly made stuff, but it drags out the buying process and ties up cash. Verifying a supplier’s quality record becomes as important as price or delivery speed. I’ve learned not to skip that part, even when paperwork looks convincing.
Price isn't stable. Raw materials for making 2-Phenylimidazole face swings as oil prices, production shutdowns, or export restrictions ripple through the market. Several years ago, a spike in environmental regulations forced some suppliers to halt operations abruptly. For anyone building production plans around bulk quantities, this can wreck cost projections.
Trying to solve this means staying in touch with more than one supplier, and not ignoring regional shifts in regulation. Early ordering and forecasting help, but sometimes nothing fixes a sudden supply chain gap except searching for a replacement compound altogether. In my experience, building strong relationships with reliable producers buys peace of mind – more than pressuring for the absolute lowest price does.
Many firms now invest in deeper audits of suppliers, asking tough questions about sourcing, sustainability, and scale-up capabilities right from the start. Doing so uncovers issues before the first truck leaves the plant. If a product is critical and no backup option exists, some bigger companies even buy up part of a supplier’s output with long-term contracts. For medium enterprises, gathering up-to-date, on-the-ground intelligence often means working with specialized sourcing agents who speak the language and visit the factories.
A bit of industry coordination wouldn’t hurt, either. Sharing baseline data about trusted suppliers and the reality of regional bottlenecks saves a world of pain down the line. Direct engagement and transparency do more for bulk buyers than any slick online catalog ever will.
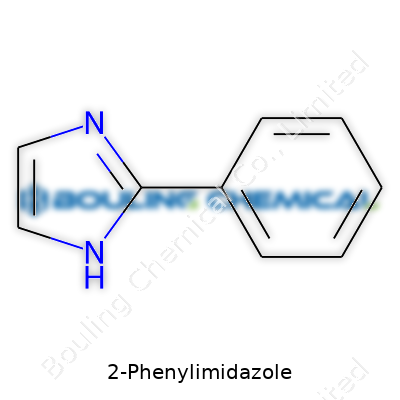

| Names | |
| Preferred IUPAC name | 1-Phenyl-1H-imidazole |
| Other names |
2-Phenylimidazole 2-Phenyl-1H-imidazole 1H-Imidazole, 2-phenyl- |
| Pronunciation | /tuː ˈfiː.nɪl ɪˈmɪd.əˌzɔːl/ |
| Identifiers | |
| CAS Number | 670-96-2 |
| Beilstein Reference | 120922 |
| ChEBI | CHEBI:18498 |
| ChEMBL | CHEMBL128962 |
| ChemSpider | 201408 |
| DrugBank | DB08347 |
| ECHA InfoCard | 100.039.347 |
| EC Number | 2236-34-4 |
| Gmelin Reference | 85780 |
| KEGG | C06596 |
| MeSH | D015746 |
| PubChem CID | 70182 |
| RTECS number | UN8250000 |
| UNII | I88YD546SA |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C9H8N2 |
| Molar mass | 144.17 g/mol |
| Appearance | White to light yellow crystalline powder |
| Odor | Odorless |
| Density | 1.112 g/cm3 |
| Solubility in water | slightly soluble |
| log P | 0.51 |
| Vapor pressure | 0.000073 hPa (25 °C) |
| Acidity (pKa) | 6.95 |
| Basicity (pKb) | 8.6 |
| Magnetic susceptibility (χ) | -5.18E-6 |
| Refractive index (nD) | 1.62 |
| Viscosity | 1.41 mPa·s (25°C) |
| Dipole moment | 2.80 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 333.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | +98.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4030 kJ/mol |
| Pharmacology | |
| ATC code | D01AE17 |
| Hazards | |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements for 2-Phenylimidazole: "P264, P280, P305+P351+P338, P337+P313 |
| Flash point | 113°C |
| Autoignition temperature | 579 °C |
| Lethal dose or concentration | LD₅₀ oral rat 960 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 600 mg/kg |
| NIOSH | BMM72 |
| PEL (Permissible) | No PEL established |
| REL (Recommended) | 50-200 mg/kg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
1-Methyl-2-phenylimidazole 2-Phenylimidazoline Benzimidazole 4-Phenylimidazole Imidazole |