Chemists started paying attention to imidazoline compounds in the early twentieth century. 2-Phenyl-2-imidazoline attracted interest almost straight away, given its blend of heterocyclic and aromatic features. The story takes off in labs that sought new medicinal scaffolds and industrial agents as companies boosted demand for better additives and intermediates. As research revealed, this compound did not sit quietly on the shelf. Patents trace back decades, pinpointing when research teams pursued imidazoline derivatives for both medical and technological breakthroughs. Chemists still draw on this early foundation of curiosity and methodical study, building richer knowledge every year.
2-Phenyl-2-imidazoline shows up as a white to off-white crystalline material with a faint amine-like scent, usually arriving in bottles marked for lab use. Chemists count on it as a building block, often as a precursor for making other sophisticated molecules or as a stabilizer in specialty formulations. The appeal isn’t just its availability—the molecule’s ring system brings unique reactivity, and its stability keeps breakdown or spoilage at bay under reasonable storage conditions. Sometimes you run across synonyms like N-Phenyl-2-imidazoline or even “phenylhydrogen-imidazoline.” Buyers should check lot numbers and purity details, since different suppliers stick to varied grades or production methods.
This compound presents itself as a stable and free-flowing solid at room temperature, melting at roughly 130-135°C. Its molecular formula, C9H10N2, hints at the aromatic ring’s weight. Insoluble in water, but soluble in most organic solvents—benzene, ether, and chloroform all do the trick—or it will dissolve in dilute acids, thanks to basic nitrogen sites. Chemists often mention its lack of volatile features under ambient conditions, contributing to its predictability in storage or handling. No wild color changes or quick decomposition when exposed to light or air in normal lab settings.
Bottles carry information about assay levels, typically showing purity at or above 98 percent by GC. Some lots include minor residual solvent content, so data sheets outline acceptable limits. Technical bulletins flag melting point, molecular weight, storage instructions (room temperature, sealed container, away from moisture), and recommended shelf life. Labels may also warn about potential hazards like eye or skin irritation, along with standard pictograms in line with GHS standards. Quality teams check for batch consistency using precise analytical reports—HPLC and NMR testing stand out as the go-to methods for confirming structure and purity.
Synthesis generally starts with phenylacetonitrile or benzaldehyde as the aromatic core source. Reacting this with ethylenediamine through either reductive amination or cyclization produces the basic imidazoline structure. Older procedures favored acidic or basic catalysis followed by neutralization, but process chemists streamlined the reaction using controlled heating and solvent selection to push yields higher. Cleanup typically ends with solvent extraction and recrystallization. The steps suit both small lab settings and plant-scale reactors, with tweaks depending on desired throughput and purity targets. Safety measures around pressure and reagent addition speed keep mishaps rare even in bulk preparation.
The structure makes it easy to introduce changes at aromatic or imidazoline nitrogen positions. Electrophilic aromatic substitution (like nitration or halogenation) opens the door to tailored analogs for specialty uses. Alkylation, acylation, and reductive amination extend the reach of the compound in medicinal chemistry. Teams building advanced ligands often attach functional groups at either phenyl or nitrogen centers. Its base character allows salt formation with a range of acids—hydrochloric acid generates a stable hydrochloride, for instance. The molecule’s ring tension also makes it react with certain oxidizers, shaping routes toward new heterocyclic scaffolds.
Chemists order this compound under many names, each tracing back to its core structure. “N-Phenyl-2-imidazoline” shows up on import paperwork or catalog entries, as does “Benzylaniline imidazoline” in some European or Asian markets. At times, suppliers shorten it to “Phenylimidazoline,” but regulatory filings usually insist on the full IUPAC form for clarity. These alternate names pop up in literature searches and patent reviews, so professionals keep an eye out for all likely spellings during research or procurement.
Defense against accidental exposure ranks high for anyone working with 2-phenyl-2-imidazoline. Even low doses can trigger irritation of eyes, skin, or mucous membranes, prompting the use of chemical-resistant gloves, splash goggles, and fume hoods. Safety data sheets point to best practices for spills or accidental contact, relying on plenty of ventilation and compatible absorbent for cleanup. Disposal follows national laws about organic chemical waste, and lab teams train for careful segregation to avoid reactive mishaps. Each shipment includes documentation for transport and hazard class so logistics never cut corners. Process operators know not to underestimate routine safety steps on the job.
Beyond its established use in chemical research, 2-phenyl-2-imidazoline finds value in pharmaceutical development, agricultural chemistry, and materials science. On the medicinal side, scientists used this structure while developing imidazoline-based drugs, testing both antihypertensive and anti-inflammatory properties. It shows up as a precursor in dye manufacture—turning up in colorant blends for plastics or textiles—and engineers look into its effectiveness in corrosion inhibitors for water systems. Other areas include rubber compounding and specialty adhesives, where its ring chemistry supports cross-linking reactions or complex stabilizer blends. Many research programs tap its versatility as a base for new ligand families or heterocyclic experimentation.
Academic labs and commercial R&D divisions keep probing the boundaries of 2-phenyl-2-imidazoline chemistry. Synthetic chemists explore ring-modified derivatives for better drug candidates, tackling both target affinity and metabolic stability. Some teams design sensors using imidazoline frameworks, harnessing electronic properties for analytical devices. Material scientists pursue advanced coatings and polymer additives by pairing this scaffold with smart monomers. Recent years brought a surge in computational modeling, accelerating design of derivatives before costly benchwork. The pace of peer-reviewed publication and patent activity ensures the knowledge base never stands still.
Laboratory studies indicate a need for caution: acute exposure causes local irritation in mammals, and researchers ran in vivo studies showing potential neurotoxic effects at high doses. Chronic studies remain sparse, but regulatory authorities generally advise against ingestion, inhalation, or repeated skin contact outside controlled environments. Toxicologists work on clarifying metabolic breakdown products and long-term bioaccumulation risks, especially relevant as applications extend into agriculture or medicine. Ongoing testing feeds into stricter workplace limits and environmental guidelines, reducing surprise liabilities as the compound’s role expands in industry.
Forward-thinking chemists and engineers recognize the promise sitting inside the 2-phenyl-2-imidazoline core. New synthetic methods open opportunities for greener production, cutting down waste or improving selectivity. Medicinal chemistry continues to chase imidazoline derivatives with improved safety or efficacy for next-generation therapeutics. Materials researchers craft fresh composites and sensors, using this molecule’s structure to gain fine control over electrical, adhesive, or catalytic properties. As markets shift and technology evolves, the compound’s adaptability ensures it stays woven into the research pipeline. Safer, cleaner, more effective processes will shape how industries source and use this chemistry, keeping scientists busy addressing both opportunity and responsibility for years ahead.
2-Phenyl-2-imidazoline doesn’t get the same headlines as big-name pharmaceuticals or miracle crop sprays, but it covers quite a bit of real-world ground. Over the years, folks in labs and factories have kept this compound in their supply racks for a few practical reasons. If you dig deep enough, you’ll see it weaving through industrial jargon and patent filings, always showing up wherever someone needs a blend of chemical punch and targeted use.
The first place I ran into this molecule was in a chemistry lab during a lesson about organic synthesis. 2-Phenyl-2-imidazoline has stood out as a starting material for building other chemicals, sometimes even medicines. Some anti-inflammatory drugs owe part of their structure to derivatives of this core. Researchers go to it when they need a basic frame that’s easy to tweak with new groups or atoms.
Beyond the flask and beaker, this compound walks straight into the textile industry. Its primary salt forms slide right into dye formulas. With so many processes leaning on fast, strong dyeing, textile workers prefer a chemical that lets dyes bite deeper into fibers. A little bit in the right spot can help colors hold tighter after dozens of washes.
Formulators in cosmetics have also grabbed onto this molecule. It has antibacterial traits, so you’ll see it in some skin creams and cleansers. Sometimes, chemists favor it for stabilizing formulas or giving preservation a boost, especially in products that spend time on store shelves.
Safety always comes up. Anyone who’s ever handled an aromatic compound in a lab knows you want gloves and a fume hood. 2-Phenyl-2-imidazoline packs a chemical bite if used carelessly. In Europe and North America, chemical inventories flag compounds like this for closer monitoring. Regulatory agencies ask for proper labeling, and end-users must control waste streams to avoid any environmental hit.
Anyone in manufacturing has stories about new rules forcing changes overnight. If the authorities suspect a compound could be harmful to workers or wildlife, they clamp down. Companies have learned to build processes that capture fumes and treat water on-site, keeping residues from slipping into rivers or leaking into the air.
Every industry faces the same challenge—stay useful but not reckless with resources. With 2-phenyl-2-imidazoline, the discovery of greener synthesis methods could land hard in the next decade. Some research groups now chase plant-based intermediates that mimic the same actions but break down faster in nature. Improving how factories handle byproducts grants more freedom to keep using what works, without letting hazards stack up.
In my view, this story always comes back to balance. Useful molecules solve actual problems, but demand respect for health and the environment. Scientists and factory workers have shared responsibility. Safety protocols, plenty of training, and a willingness to swap out an old method for something better ensure progress doesn’t come at too high a price.
Anyone who spends time around chemicals like 2-Phenyl-2-Imidazoline needs to treat the job with respect. I’ve handled plenty of chemicals with mouthful names, and the best way to avoid accidents starts with understanding what you’re dealing with. This compound shows up in labs, sometimes in specialty manufacturing, and maybe even as a reagent, but it can cause real trouble for anyone who gets careless around it.
Every time I’m about to open a container of 2-Phenyl-2-Imidazoline, I remind myself that skin, eye, and airway contact brings quick irritation. Just a small spill on your hands or a whiff of the vapor feels like trouble. The chemical isn’t just an irritant—prolonged exposure ramps up health risks, especially in small, enclosed rooms. If you’ve ever busted out a bottle without gloves or a mask and felt your eyes or throat burn, you know what I mean.
The importance of proper equipment isn’t up for debate. Nitrile gloves, full goggles, and a decent lab coat put up a strong first line of defense. Cotton clothes help, but synthetics run the risk of melting if things go badly. I’ve seen people reach for latex gloves out of habit, but they don't give reliable protection here. The right gloves and eye shields keep you out of the emergency wash station and away from the clinic.
I’ve never liked handling volatile chemicals in a cramped, airless space. 2-Phenyl-2-Imidazoline may not smell nasty enough to chase you out, but fumes build up before you realize. Hoods with real airflow, not just a whirring desk fan, pull vapors and dust away from your face. If you work outside of a fume hood, you play roulette with your health. Proper ventilation helps cut down on headaches and respiratory irritation, and it’s the one lab feature you’ll miss most if you skip it.
Every time I’ve stored 2-Phenyl-2-Imidazoline, dryness becomes a priority. Moisture or high heat speeds up degradation and invites leaks. Sealed bottles with a tight-fitting lid (ideally a chemical-resistant type) go straight into a cool, dry spot. Forgetting this can set you up for weird odors, ruined samples, or even pressure build-up. Mark bottles with clear hazard labels so nobody mistakes this for something innocent.
I’m all for confidence at work, but keeping a bath and an eyewash station within arm’s reach saves from panic. Any splash, no matter how small, deserves a rinse—fifteen minutes’ worth, not just a splash-and-go. If someone breathes in dust or vapors, get to clean air as quick as possible, keep calm, and see a medical pro without stalling. Having the right contacts and clear steps posted makes a real difference.
I’ve seen the best lab gear let people down if folks don’t know how to use it. Hands-on, practical training builds habits that sink in. Walk-throughs for mixing, pouring, storing, and cleanup stick better than any online slideshow. People remember real spills, even if staged, and pick up the muscle memory that beats written rules every time. Lab safety isn’t just a paper exercise—it comes from doing things right, alongside people you trust.
Hunters for straightforward answers often hit a wall when chemistry gets splashed with technical terms, but some molecules deserve a closer look. Take 2-Phenyl-2-Imidazoline—the ring system feels a bit like a lock, holding back complexity until you look at how the pieces fit together. The molecule carries an aromatic benzene ring thrown onto a five-membered imidazoline core. The structure matters because it gives the compound much of its chemical character, affecting both reaction and application.
Let's break down the way 2-Phenyl-2-Imidazoline comes together. At the center sits an imidazoline ring—a five-membered structure with two nitrogen atoms at positions one and three, and three carbons forming the rest of the ring. At the two position, a phenyl ring (the kind found in benzene) attaches, adding extra stability and a handle for chemical properties. The skeleton looks like this:
Glue these together at the right spot, and you get a molecular formula of C9H10N2. The structure stacks these elements into a compact, somewhat planar arrangement. Drawing it on paper, most chemists recognize the N—C—N segment in the ring attached directly to a benzene.
Walking through a lab, I often stopped at shelves filled with heterocycles like this one. These compounds end up in a range of products, pharmaceuticals, and materials. That phenyl group isn't just decoration. It locks in extra stability and delivers a useful anchor for further modifications. The imidazoline ring, with its two nitrogens, lets it play with hydrogen bonds and stick to other molecules in a way rings without nitrogens can’t. Scientists look at 2-Phenyl-2-Imidazoline as a platform: start here and you gain access to dozens of more complex chemicals downstream.
With great utility comes the need for care. The ring system and the phenyl group both carry risks. Exposure at the bench sometimes brings irritation or toxicity, a lesson learned after watching colleagues work without proper protection. The molecule doesn’t belong near unprotected skin or eyes. Clear labeling and locked storage stop it from becoming a risk to health or the environment. Safe handling practices, written down and discussed in the lab, keep accidents out of the daily routine.
Chemists and industry managers turn toward greener processes each year, steering workflows away from solvents and steps that increase hazards. Open access to safety sheets and simple instructions in local languages can help staff understand the risks and act before mishaps. Sharing experiences—mistakes and successes alike—across teams brings fresh shortcuts and sheds light on safer substitutes when the hazard outweighs the benefit.
Getting to know 2-Phenyl-2-Imidazoline means more than just reciting its formula. The piece-by-piece construction of its aromatic ring and five-membered core reveals why it behaves the way it does. This knowledge guides both its practical use and the care needed to handle it right. Chemistry stays approachable when explanations stick to real examples and honest experiences rather than wrapping up in textbook language.
Anyone stepping into a laboratory knows the frustration that comes when a promising reagent just refuses to dissolve. Eventually, “solubility” becomes more than a technical checkmark. The fate of a project hinges on the simple question: will this go into solution? With 2-Phenyl-2-Imidazoline—a molecule that has found fans in analytical chemistry and industrial settings—the urge to clarify its solubility comes up often and with good reason.
I’ve spent afternoons coaxing stubborn compounds into solvents, and imidazoline derivatives rarely go quietly. 2-Phenyl-2-Imidazoline, with its aromatic ring and polar imidazoline group, splits its allegiance. In my bench experience and after combing through chemical catalogues and primary data, water doesn’t take kindly to large, hydrophobic groups like phenyl. The result: very limited water solubility, as many academic sources confirm. Stirring, heating, sonication—it rarely budges. Most find a cloudy suspension rather than clear solution.
This changes with organic solvents. Toluene, chloroform, and even ethanol often rescue the situation. In various synthetic protocols, researchers dissolve 2-Phenyl-2-Imidazoline in ethanol or similar organic solvents without issue. The compound’s phenyl ring pairs well with nonpolar or slightly polar substances, so acetone, methanol, and DMSO become solvents of choice for clean solutions. I’ve worked side-by-side with students frustrated by endless shaking in water, their spirits lifting when the switch to ethanol finally does the trick.
The confusion doesn’t just spring from mixed anecdotes. Labeling on bottles sometimes omits direct solubility data. Articles copy information from old “standard references” without testing in modern environments. Dissolving conditions—temperature, mixing—get left out, making reproduction of results tricky. Many laboratories also lack the luxury of screening a dozen potential solvents, so decisions come down to habit instead of hard evidence.
The wrong solvent means poor yields, delays, and wasted resources. Imagine planning a purification, expecting your compound to stay in the aqueous phase, only to find it stuck with the organics. Even more important, solvent choice ties into safety. Nonpolar solvents bring flammability and regulatory headaches. Using DMSO or methanol means more steps to protect skin and eyes. Chemistry textbooks might glaze over this, but practical lab work demands respect for these everyday challenges.
Solubility questions rarely get answered once and for all. Batch impurities, ambient temperature, or a tiny change in pH can nudge results. Researchers benefit from running small-scale solubility tests using their actual reagent and real-world solvents. I recommend keeping a lab notebook with direct observations—cloudy, clear, needs heat, dissolves fast—and not shying away from a quick literature search for updates.
Suppliers can also do more. Reporting actual solubility ranges, with clear context for experiments, helps more than vague statements. If more of us shared detailed methods in papers and lab notes, the collective knowledge grows and mistakes shrink.
For anyone counting on 2-Phenyl-2-Imidazoline, organic solvents usually promise better solubility and smoother workflows. Choosing the right one brings down risks, saves time, and protects budgets. Testing and documentation keep teams aligned. Bottom line: asking the old solubility question, and getting a real answer, never goes out of style.
Every lab worker who handles organic chemicals learns one thing quickly: safe storage means fewer headaches, less waste, and lower risk. 2-Phenyl-2-imidazoline—used in everything from chemical synthesis to research—demands the same respect. I’ve seen a few wasted batches over the years due to poor storage, so I watch out for the basics every single time.
This compound shows decent stability in its pure solid form, but things can go south fast with too much humidity or direct light. I once found a bottle left near a sunny window. The color changed and a faint smell drifted off—a clear warning that light and oxygen have already kicked off unwanted changes.
Direct sunlight jump-starts chemical breakdown in many organic molecules. Keeping 2-Phenyl-2-imidazoline in a tightly sealed, light-blocking container protects its molecular structure. Opaque or amber glass bottles with screw-top lids usually work best. Plastic may not hold up, especially with older solvent bottles leaching plasticizers which could contaminate your sample.
The best lab managers I know treat every chemical with a kind of respect. I’ve never seen them stash sensitive materials beside heaters or refrigeration units that cycle temperatures up and down through the day. 2-Phenyl-2-imidazoline keeps best at room temperature, in a place that stays steady—think 15–25°C (59–77°F) without sudden spikes.
Don’t freeze this stuff unless the manufacturer specifically calls for sub-zero storage. Freezing can change the crystal structure and may even trap moisture inside a container as ice. Thawing can make things worse—the compound attracts moisture quickly once it’s opened back up.
I’ve seen careless handling break the bank. Once, a coworker left the lid loose. 2-Phenyl-2-imidazoline readily absorbs moisture from the air, turning clumpy and less pure. Water can trigger unwanted side reactions, especially if you’re relying on it for research with tight purity specs.
Desiccators filled with drying agents like silica gel or anhydrous calcium chloride minimize moisture exposure. This extra step pays off, especially during humid months. Small labs sometimes skip this to save time, but the result can be unreliable experiments or failed syntheses.
Sloppy labeling causes confusion and mistakes. Every fresh container should be labeled with date received, date opened, and expiry if available. I’ve walked into labs where old unmarked bottles collect dust in the back—no one knows if they’re still good or not. Clean logs and clear labels keep everyone safe and projects moving forward.
Safety isn’t just a checkbox. Good storage habits protect your health and save money by keeping chemicals reliable. Always use chemical-grade PPE when handling powders or making transfers. Spills and contamination delay work and put people at risk. I’ve found that even seasoned chemists skip steps when they’re in a hurry, leading to accidents that should have never happened.
In short, keep 2-Phenyl-2-imidazoline cool, dry, away from light, and tightly sealed. A little extra care up front saves time, money, and headaches down the line. The old hands in any lab know: treat your chemicals right, and they’ll do the job you expect every time.
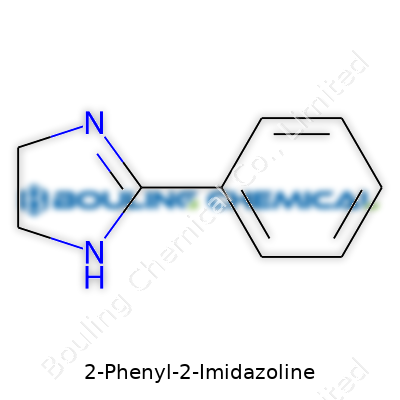
| Names | |
| Preferred IUPAC name | 2-phenyl-4,5-dihydro-1H-imidazole |
| Other names |
2-Phenylimidazoline Phenyltolimidine 2-Phenyl-4,5-dihydro-1H-imidazole Nafazoline base Tolazoline |
| Pronunciation | /tuː-ˈfiː.nɪl-tuː-ɪˌmɪd.əˈzoʊ.liːn/ |
| Identifiers | |
| CAS Number | 930-50-7 |
| Beilstein Reference | 120873 |
| ChEBI | CHEBI:36158 |
| ChEMBL | CHEMBL15110 |
| ChemSpider | 12029 |
| DrugBank | DB08225 |
| ECHA InfoCard | 14a5f44d-fbb7-4927-b39c-9d048b90faaa |
| EC Number | 220-204-8 |
| Gmelin Reference | 146361 |
| KEGG | C06539 |
| MeSH | D015315 |
| PubChem CID | 70303 |
| RTECS number | NR1575000 |
| UNII | SYR4Q7B9HZ |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DJ5C48O2L5 |
| Properties | |
| Chemical formula | C9H10N2 |
| Molar mass | 146.19 g/mol |
| Appearance | White to yellowish crystalline powder |
| Odor | aromatic |
| Density | 1.09 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.56 |
| Vapor pressure | 0.0566 mmHg at 25°C |
| Acidity (pKa) | 13.24 |
| Basicity (pKb) | 7.55 |
| Magnetic susceptibility (χ) | -66.42×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.602 |
| Viscosity | Viscous liquid |
| Dipole moment | 4.08 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 330.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 90.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3855 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | C01CA13 |
| Hazards | |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "H302, H314, H410 |
| Precautionary statements | Precautionary statements: P261, P305+P351+P338, P312 |
| Flash point | 104.4°C (string) |
| Autoignition temperature | 495 °C |
| Lethal dose or concentration | LD50 oral rat 220 mg/kg |
| LD50 (median dose) | LD50 (median dose): 500 mg/kg (oral, mouse) |
| NIOSH | MW4800000 |
| PEL (Permissible) | PEL (Permissible): Not established |
| REL (Recommended) | REL: NIOSH REL 2 mg/m³ (skin) |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Oxymetazoline Xylometazoline Tetrahydrozoline Naphazoline Tolazoline |