Chemists in the early 20th century started tinkering with piperazine structures, searching for new compounds with promising activity. 2-Oxopiperazine entered scientific discussions as researchers carved out families of heterocycles, tracking how small tweaks could shape molecular behavior. The journey saw the compound filter through pharmaceutical applications and chemical catalogs, always with fresh eyes on tailoring its backbone for everything from antibiotics to enzyme inhibitors. Sourcing the reliable literature, one sees the steady march from dusty journals in the 1950s to today's advanced patents and biotech pipelines, with 2-oxopiperazine’s name popping up often enough to prove its stubborn usefulness.
Every chemist who works with building blocks for drug discovery has run across 2-Oxopiperazine. This six-membered ring, capped with a ketone at the 2-position, finds a spot in libraries aimed at new therapeutics and serves as a staple intermediate. Its structure encourages curiosity because it offers a blend of rigidity and reactivity, showing up as a pivotal core or as a subtle side-chain. You'll find it listed in supplier catalogs for research, each vial destined for countless synthetic adventures. People in the lab see it as a reliable starting point, not just for one niche market, but for broad explorations: pharmaceuticals, material science, agrochemicals, and sometimes even dyes.
If you pick up a sample, you're most likely to see a white crystalline solid, odorless and easy to handle. The melting point sits near 115-120°C, with solubility letting it dissolve nicely in dimethyl sulfoxide or methanol but not so much in water. Its molecular formula is C4H6N2O, weighing in at about 98 g/mol. It holds up to mild heating, yet the ring opens under strong acid or base, so handling with basic lab safety makes for smooth sailing. Chemically, the lactam carbonyl draws nucleophiles, and the nitrogen atoms lend the molecule versatility, both for further reactions and as a seat for new approaches in synthesis.
Labs order the compound based on purity, with research purposes demanding grades above 98%. The certificate of analysis typically lists melting point, water content, and organic purity by HPLC or GC. Packaging labels must show the CAS number (505-22-6), batch number, and hazard pictograms. Labs keep eye wash and gloves handy, though the compound sits low on most hazard scales. Regulatory guidance from REACH or GHS standards gets reflected on every shipping box and data sheet, so anyone in the workflow sees the same information, whether they're behind a bench or in procurement.
Synthesis options split into two main routes. For small batches, cyclization of suitable diamines with phosgene substitutes like triphosgene makes a reliable path toward the ring. For those wary of hazardous reagents, condensation of ethylenediamine with glyoxal or its derivatives followed by oxidation can bring about the same core. Scale-up chemists adjust conditions to minimize side products, opting for anhydrous media and tight temperature controls. It’s hard not to appreciate the balance in the synthesis: quick if you need just a gram, yet scalable enough for process development. Experience shows yields often push 75% or better, with minimal headaches from purification.
The lactam carbonyl serves as a launch pad for transformations: alkylation, acylation, or nucleophilic substitution. Reductive amination swaps in fresh sidechains, letting chemists decorate both nitrogens. The ring stays rigid, giving structure to larger molecules. You see modifications where arylation of nitrogens leads to new pharmacophores, or where reduction opens the door to piperazine analogues with altered reactivity. If someone wants to shuffle functionality, they might oxidize it further or bring in protecting group chemistry. Each adjustment ripples through the molecule’s activity, offering up fresh analogs for screening in biological assays. It’s this chameleon-like quality that keeps it relevant.
In supplier catalogs and chemistry papers, it's called 2-Oxopiperazine, 2-Piperazinone, or Piperazine-2-one. CAS 505-22-6 tags it unmistakably. Brand names from chemical vendors might attach their own twists—Sigma-Aldrich or TCI both list it with matching structural diagrams, sometimes offering hydrochloride salts or substituted variants. Synonyms can cause headaches when ordering, so chemists learn to cross-check, cross-reference, and always review the structure in the fine print.
Lab routines for 2-Oxopiperazine echo those for most fine organic chemicals: gloves, goggles, and plenty of ventilation. MSDS sheets flag mild irritation risk for eyes and skin, yet acute toxicity remains low according to REACH and GHS classifications. Handling larger quantities calls for careful dust control and avoidance of ignition sources, primarily because the powder can become airborne during weighing. Alcohol spills or accidental inhalation aren’t common, but it pays to follow institutional guidelines, store in a dry cool spot, and log inventory in compliance software. Disposal routes favor incineration or solvent washout under chemical waste guidelines.
Most action takes place in drug discovery—chemists reach for 2-Oxopiperazine as they design kinase inhibitors, enzyme inhibitors, and scaffolds for antimicrobial agents. The compound’s structure fits snugly into enzyme active sites, sparking interest for both modulating biological targets and fine-tuning drug-like properties. In a handful of materials science directions, it anchors specialty polymers or serves as a crosslinker. Synthetic routes into agrochemical agents often build on its profile, searching for new ways to disrupt pest metabolism. Patent searches turn up a steady stream of diverse applications, but the core remains small-molecule therapeutics, a reflection of both the ring’s adaptability and medicinal chemistry’s hunger for new shapes.
Recent research ties 2-Oxopiperazine to several hot pharmaceutical targets. Teams dig into its role for oral bioavailability, metabolite stability, and receptor selectivity. Templated approaches through combinatorial libraries let this ring join a dizzying array of candidates, with medicinal chemists optimizing substitutions for both safety and activity. The hydrogen-bonding network created by the carbonyl and nitrogens offers unique interactions within biological targets, and gains traction in modeling studies for structure-activity relationship work. Its toolkit value keeps it featured at conferences, especially as new analytical and computational approaches unlock more secrets of its chemical behavior and biological action.
Toxicity profiles, to date, raise few red flags. Animal studies at standard doses report low acute toxicity, no significant carcinogenic or teratogenic effects, and rapid clearance from the body. Labs keep track of chronic exposure risks, but few incidents make appearances in case studies or workplace logs. Biological assays focus more on derivatives since actual therapeutic compounds often embellish the base structure with fresh chemical arms. Environmental impact assessments flag minimal bioaccumulation. Regulatory filings remain straightforward, but chemists continue to check each analog through both in vitro and in vivo screens before moving toward clinical candidates.
Trends in medicinal chemistry point to continued exploration of 2-Oxopiperazine for both new treatments and as a backbone for more complex molecules. Demand links directly to how well the scaffold adapts to tighter selectivity requirements in drug targets and the always-moving challenge of resistance in infectious disease. Automation in high-throughput screening and AI-based design both expand the landscape for follow-up. Greener synthesis, safer reagents, and more sustainable supply chains will likely shape the next generation of production, making it not just a reliable workhorse, but a smarter one. The story of 2-Oxopiperazine keeps echoing because of its ability to evolve alongside technology, always offering up new wrinkles for creative minds in the lab.
Ask almost anyone outside a chemistry lab about 2-Oxopiperazine and you’ll probably get a blank stare. This compound doesn’t make headlines, but it works hard behind the scenes in the pharmaceutical and chemical industries. I’ve spent plenty of time helping science majors prep for exams, and every once in a while, 2-Oxopiperazine comes up. It’s not flashy, yet its story is packed with quiet importance.
Anyone who’s taken a strong antibiotic or goes through cancer therapy should know a bit about the stuff that goes into their meds. 2-Oxopiperazine has turned out to be a building block for making these drugs. This cyclic compound—picture a six-membered ring with nitrogen and oxygen at the right spots—forms the backbone for creating all sorts of molecules in drug labs. Chemists build off it the way carpenters frame out houses, turning it into things like antihypertensive agents, HIV treatments, antifungal agents, and anti-cancer drugs.
You may recall hearing about Atazanavir, a drug used in HIV therapy. Its synthesis owes plenty to the framework provided by 2-Oxopiperazine. This chemical makes complicated molecular structures easier to assemble, daisy-chaining onto other molecules and opening doors for researchers. That’s not just a technical footnote—it means treatments arrive sooner on pharmacy shelves, sometimes shaving years off development cycles.
Away from the pharmacist’s counter, 2-Oxopiperazine finds its way into materials that touch everyday life. Companies that design speciality polymers often rely on it to create flexible, tough plastics. These polymers could show up in medical devices or coatings on wires. My cousin, who works in electrical engineering, once pointed out that some insulating layers on high-performance cables start with compounds produced using 2-Oxopiperazine. Additives like this can make materials last longer—one of those small victories in the war against planned obsolescence.
Agricultural researchers see value here too. Some pesticides and herbicides are developed using 2-Oxopiperazine as an early step. These chemicals can help protect crops from destructive fungi or weeds, which ties right back to the food on our tables.
Everyone wants safer, more effective drugs, but the chemistry needed to make them can cause headaches. With 2-Oxopiperazine, the challenge lies in keeping manufacturing clean and safe. Chemical reactions for making it often use solvents or catalysts that end up as hazardous waste. In my time volunteering at a local science center, conversations about these by-products opened my eyes: companies walk a tightrope between producing lifesaving medicine and managing tricky leftovers that could harm the environment.
Some green chemistry initiatives have made progress swapping out toxic reagents for safer options. Researchers test alternative reaction pathways, looking for ways to keep production clean without giving up efficiency. More universities build sustainability into their chemistry programs, so tomorrow’s scientists will have tools that respect both health and the planet.
2-Oxopiperazine might never get the spotlight, but its footprint touches a surprising number of lives. Whether it’s inside a pill bottle or the lining of a cable, this unassuming compound helps connect breakthroughs in the lab to the real world.
Talking chemistry in everyday terms isn’t always easy, but looking at 2-Oxopiperazine, things actually start to make sense if you break them down. Here’s the deal: the molecule's structure looks like a six-membered ring with two nitrogens in the ring and a keto (=O) group next to one of them. Chemists write it as C4H8N2O. CAS numbers give us a way to always refer to the same chemical, no matter which supermarket of science you’re shopping in. For 2-Oxopiperazine, the CAS number is 505-22-6. I kept a battered CRC Handbook from my grad school days, and this number was my shortcut when sifting through pages and pages of similar molecules.
A lot of what happens in those glass beakers and reactors across the world actually depends on small, almost forgotten building blocks like this one. In pharmaceutical labs, 2-Oxopiperazine is a hardworking intermediate. People need drugs with specific properties, and this ring system sneaks its way into the blueprints of plenty of approved medicines. It’s compact, versatile, and chemists see it as a sort of Lego block—hooking to all sorts of other pieces.
Out of all the molecules that run through labs, the reason this one gets attention boils down to what happens once scientists slap different chemical groups onto its nitrogens. Suddenly, new drugs fighting infections or conditions such as diabetes or cancer appear on the horizon. For me, it highlighted the real world behind a string of atoms: someone running a test on this molecule today might be laying the groundwork for a treatment years from now.
Years ago, I watched a colleague work through a synthesis using 2-Oxopiperazine as a core. One contaminant ruined a whole batch, wasting days. Turns out, getting pure material isn’t just a matter of picking up a catalog and clicking. Sources matter. Some suppliers charge a premium for high-quality product, and even the method—batch or continuous—can change what you get. Labs needing a reliable supply for lots of experiments learn quickly which brands deliver clean material—and which do not.
This shifts the conversation outside of scientific circles, too. So many companies are scrambling to find new antibiotics and antivirals. Intellectual property fights sometimes hinge on which small molecules a company can make at scale and with purity. The demand for basic materials reveals pretty quickly where international supply chains are stretched or broken. Anyone who’s ever waited for weeks for a simple chemical knows how real this frustration can get.
Seasoned researchers and newcomers both agree on one point: reproducibility starts with knowing exactly what you’re using. Companies labeling 2-Oxopiperazine clearly, batch testing, and being transparent about impurities save headaches down the line. Investment in more efficient, cleaner synthesis can put smaller labs on an even playing field with giants. Fixing international supply—maybe through partnerships or clearer regulations—translates to fewer delays and more trust.
Sometimes, the molecules that sound obscure backup the breakthroughs that grab headlines. 2-Oxopiperazine, with a name only a chemist could love, quietly does the heavy lifting. For anyone passionate about making discovery real, respect for the building blocks is where progress begins.
Walking into any chemical lab, purity charts catch the eye. A bottle labeled “2-Oxopiperazine” isn’t just a bottle; it’s a silent promise about what’s inside. Chemists like having the real story, because a small percentage of impurity can throw a whole experiment under the bus.
2-Oxopiperazine wears a lot of hats these days, with roles in pharmaceutical research and also as a building block for materials or specialty chemicals. Just a pinch of unwanted material can spell wasted money or failed drug candidates, something labs want to avoid like the plague. In research, many go straight for analytical- or reagent-grade because it makes life easier and results more honest. Pharmaceutical companies push for even higher thresholds—think 98% or above—since the law and patient safety demand it.
During my time in research, I saw first-hand the difference between so-called “technical” and “high purity” chemicals. Technical grade works for rugged, large-scale manufacturing where absolute purity doesn’t matter much. It’s cheaper and usually has more leftover bits from the production process. Not all applications blink at this—say you’re making a polymer that will go into a shoe sole. Minor leftovers melt away into the larger mix.
Once you step into pharmaceutical or advanced polymer labs, every decimal counts. The fine stuff—reagent or HPLC grade—comes with tight paperwork, detailed analysis, and price tags that sometimes make you wince. But clean data, reliable drug testing, and regulatory compliance leave no room for second-guessing what’s dissolved in the flask.
Suppliers and labs typically use purity analysis methods such as HPLC, NMR, or titration. Each batch ends up with a certificate of analysis spelling out the numbers. If you ask around, most commercial suppliers offer at least two flavors: something near 98% for high-stakes work, and something in the mid-90s or low-90s for routine or bulk uses.
Early in my career, I learned the hard way about cross-contamination and how a bargain-bin purchase could nudge an entire research project off course. Many labs become loyal to suppliers whose paperwork checks out, and who don’t cut corners behind closed doors. If someone’s work only needs a mid-purity product, they’ll go that route, but nearly every researcher I know opts for higher purity once budgets allow.
Chemical access isn’t just about purity, but also price tags, logistics, and ethical sourcing. The gap between technical and high-purity grades seems to widen as regulatory walls go up. One solution is to pressure manufacturers for more transparent tracking, so that every buyer knows exactly what impurities ride along.
Governments and academic consortia can also set industry-wide standards more clearly—maybe even offer platforms for chemical recycling or purification to make better grades more widely available. From what I’ve seen, small labs sometimes pool resources to buy in bulk and purify what they need, skipping expensive middlemen.
Anyone who has worked shoulder-to-shoulder with a nervous grad student knows the stress of doubting what’s in a vial. Clearer grading, honest supplier relationships, and open discussions between labs and vendors all make a real difference in making sure that a bottle of 2-Oxopiperazine is exactly what it claims to be.
With so many chemicals in the lab, few smack you quite as quickly as 2-Oxopiperazine if you let your guard drop. As someone who’s stood in front of shelves packed with jars and bottles—sometimes labeled in permanent marker, sometimes not—what really matters isn’t a stack of paperwork or a warning on the side. It’s the stuff you do every day: where you keep it, how you move it, and whether you’re the kind of person who cuts corners.
2-Oxopiperazine stays stable in a dry, cool spot, out of the sun’s reach. Heat messes with it, so leaving it next to radiators or tucked in the path of sunlight only courts trouble. I’ve learned to clear a low-traffic shelf for these jars, away from acids, bases, and anything that might start a fire. Damp shelves? Forget them. Humidity knocks this chemical around, and once moisture creeps in, you can’t trust the rest of the container.
Many storerooms still ignore ventilation. Open up one of these chemicals in a cramped space, your eyes water, and you know you just lost some brain cells. Dedicating a section of a ventilated cabinet—one with a solid lock—gives you more than peace of mind. It’s knowing there’s a barrier between you and an emergency call to environmental health and safety.
2-Oxopiperazine prefers containers that don't easily react—good glass or top-grade plastic. I once saw a guy try to salvage a batch stored in a cracked bottle. Good luck with that. Any spill becomes an uncontrolled mess. The seal on a bottle makes a real difference since air brings in moisture. Bags or thin plastics just don't cut it; you want something thick, leakproof, and clearly labeled.
Wearing gloves and goggles isn’t just a check-the-box activity. Splash a little 2-Oxopiperazine on your skin, and you’ll regret ever thinking about shortcuts. Use a scoop or spatula—nobody enjoys those awkward calls to poison control. Ventilation cuts down on dust and vapors, which hang around longer than you’d like. Fume hoods aren’t a luxury; they’re essential if you’re weighing or transferring this stuff.
I’ve worked in places where chemical waste turns up in coffee cups. Don’t do that. Dedicated disposal containers—clearly marked and sealed—save you and the janitor a lot of headaches. Never pour leftover material down the drain, even if you think the city water plant can handle it. That kind of attitude ruins reputations and budgets.
Keep emergency showers and eyewash stations close. If someone still gets it wrong—spills a bit or catches some in their eye—quick action makes a massive difference. Supervisors who run regular safety drills see fewer injuries for a reason. Real training beats a stack of safety binders any day.
Accidents with 2-Oxopiperazine don’t just happen to newcomers. Fatigue creeps in, shortcuts tempt seasoned hands. The best advice I ever got: act like someone’s watching, even if nobody is. Storage and handling become habits, and that’s what keeps the lab running and the people around you healthy.
Most people outside of labs have never even heard of 2-Oxopiperazine. The name alone feels ripped from a chemistry textbook. In reality, for anyone working with specialty chemicals—in research, pharmaceuticals, or chemical manufacturing—this compound is pretty familiar, usually as an intermediate for more complex molecules. People often picture laboratories as shiny, white rooms, but the truth is, even routine research deals with a rotating lineup of synthetic chemicals. Some of them pack more of a punch than others, and 2-Oxopiperazine shouldn’t be waved off as innocent just because it isn’t a household name.
On paper, 2-Oxopiperazine doesn’t rank among the top industrial toxicants. You won’t find it in mainstream news headlines. But splash some on your skin or forget to wear a mask, and you’ll discover firsthand why safety datasheets fill up with warning icons. What makes this compound tricky is its tendency to irritate. Inhalation can trigger upper respiratory discomfort. Even brief skin or eye contact creates redness and pain for some people—imagine spilling it while distracted, then trying to get through the day with burning knuckles. Long-term exposure studies are sparse, but just because data is thin doesn’t mean folks should cut corners.
Sitting through chemical safety training my first week on the job, I didn’t pay much attention until a colleague told me about an incident years back. They had rushed—skipped the gloves, assumed handling a small volume "wasn’t a big deal." By lunch, their hands itched and burned, the skin pink and blotchy. I realized those datasheets weren’t joke material. Even chemicals that aren’t flagged as “dangerous” end up causing a lot of visits to the nurse’s station if ignored.
People love to hunt for shortcuts or clever gear that lets them relax around chemicals. The real answer looks dull: stick to protective basics and respect the risk. For 2-Oxopiperazine, gloves, goggles, and a reliable lab coat form the main shield against contact—no need for fancy alternatives. Ventilation matters more than folks think. Fume hoods can seem loud or inconvenient, but minute-for-minute, they prevent accidental inhalation better than any mask. Clean hands before eating lunch or touching your face; it sounds simple, though you’d be shocked how many veterans get lax and later regret it.
Storing chemicals on the right shelf keeps accidents from snowballing. 2-Oxopiperazine should stay in well-sealed bottles, away from incompatible acids or bases. It reacts if mixed with certain strong chemicals, so never grab an unlabeled bottle in a rush. Disposal gets ignored most often. Some dump unwanted solutions down the drain, but that sends the problem downstream—to workers at water treatment plants or into the local ecosystem. Special waste containers exist for a reason: once full, professionals haul them away for neutralization, not just “out of sight, out of mind.”
Casual habits turn into industry standards. If safety routines stay consistent, they shield people who share the same workspace down the line. A culture that makes safety second nature—checking labels, wearing gloves, using hoods—lowers everyone’s risk, not just the newest intern or the strictest supervisor. Acting with care goes a lot further than trusting fate or luck.
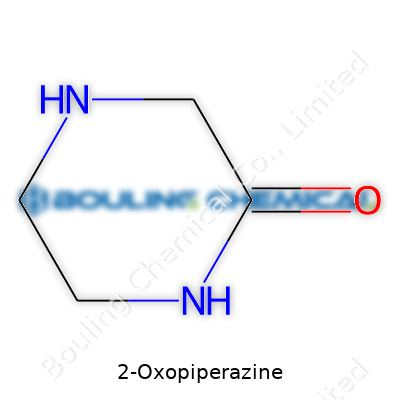
| Names | |
| Preferred IUPAC name | Piperazine-2-one |
| Other names |
2-Ketopiperazine Piperazine-2-one Piperazin-2-one |
| Pronunciation | /tuː-ˌɒk.soʊ.pɪˈpɛr.ə.ziːn/ |
| Identifiers | |
| CAS Number | 4968-01-4 |
| 3D model (JSmol) | `3D36C1DCEB1B92A3E7B5B00A2992938B` |
| Beilstein Reference | 136334 |
| ChEBI | CHEBI:73641 |
| ChEMBL | CHEMBL131056 |
| ChemSpider | 54670 |
| DrugBank | DB08319 |
| ECHA InfoCard | 03b146af-9e8d-446f-a273-1c3265c7f4e7 |
| EC Number | 211-746-3 |
| Gmelin Reference | 113629 |
| KEGG | C07434 |
| MeSH | D010079 |
| PubChem CID | 11815 |
| RTECS number | TK3150000 |
| UNII | KGJ8T0JTS1 |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C4H8N2O |
| Molar mass | • 114.14 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.18 g/cm3 |
| Solubility in water | Soluble |
| log P | -1.28 |
| Vapor pressure | 0.0386 mmHg at 25°C |
| Acidity (pKa) | 8.29 |
| Basicity (pKb) | 5.62 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.541 |
| Dipole moment | 3.57 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 385.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -467.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | Std enthalpy of combustion (ΔcH⦵298) of 2-Oxopiperazine: -3241 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed or inhaled. Causes skin and eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H319 |
| Precautionary statements | Precautionary statements for 2-Oxopiperazine (according to GHS classification): "P261, P264, P271, P304+P340, P312" Let me know if you need the full text of each code! |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 128°C |
| Autoignition temperature | 260°C |
| Lethal dose or concentration | LD50 (oral, rat): 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 2-Oxopiperazine: "370 mg/kg (rat, oral) |
| NIOSH | MW7280000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 20–25°C |