Back in the days when the study of thiophenes started gaining traction, nobody quite expected the broad reach of 2-Oxo-5-Methylthiophene within both research and industry. Scientists in the late 19th and early 20th centuries were drawn to sulfur-containing heterocycles mostly out of curiosity. By digging into coal tar and exploring its bewildering cocktail of aromatic compounds, early chemists noted the curious ways sulfur atoms change the behavior of carbon frameworks. Decades of incremental work built the foundation for how specialists now view functionalized thiophenes like 2-Oxo-5-Methylthiophene. As organic synthesis matured, laboratories around Europe and North America tried out various substitutions on the classic thiophene ring, stumbling upon methylthienones that showed real promise both academically and commercially. Looking back, it's fascinating to see how persistent experimentation and a pinch of serendipity led to a compound now heavily referenced not only in synthetic labs but also in medicinal chemistry journals.
Digging into 2-Oxo-5-Methylthiophene, you'll see a molecule that bridges basic synthetic chemistry and targeted applications. Its five-membered ring blends methyl substitution and a ketone group, providing versatility for downstream modification. It rarely belongs on the front page of chemical catalogs, yet it quietly powers a host of reactions in both pharmaceutical research and advanced materials. Its solid state at room temperature, moderate volatility, and compatibility with standard organic solvents lend practical value in both benchtop prep and large-batch manufacturing. Unlike many more exotic heterocycles, its production scale and cost rarely deter a research group with a middle-of-the-road budget.
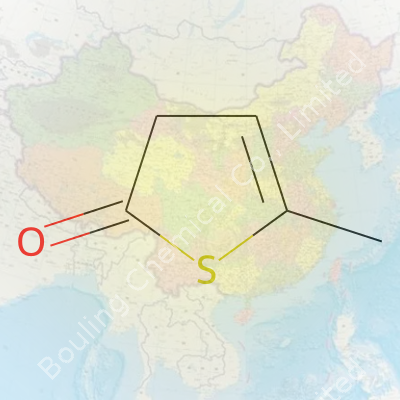
This compound carries a tightly packed five-carbon, sulfur-containing ring. The ketone at the 2-position elevates its polarity compared to unsubstituted thiophene. The methyl at position 5 tweaks the electron distribution just enough to impact reactivity. Melting around 25–35°C, it sometimes appears as a crystalline solid but often softens at a warm room temp. With slight solubility in water but greater compatibility with ethanol, ether, and common organic solvents, the material brings manageable logistics into both reaction and purification steps. A molecular weight in the neighborhood of 114 g/mol means careful dosing on the bench, but nothing daunting for the practiced hand.
Bottles of 2-Oxo-5-Methylthiophene often present as amber vials or drums clearly marked with hazard notices and batch information. Purity grades run from research-lab 95% levels to preparative work above 99%. Labels include CAS numbers (13679-70-4), recommended storage below 25°C, and warnings for both skin contact and inhalation. Trace-level impurities such as other methylthiophenones or leftover starting materials get flagged in quality-control sheets. SDS sheets highlight the chemical’s sensitivity to strong oxidants and the mild sulfurous odor. Reputable suppliers note batch date and lot, ensuring traceability for any work that might wind up peer-reviewed or patented.
The synthesis draws on a toolbox stretching back to classic Friedel-Crafts chemistry and more recent catalytic oxidation methods. Some routes start from methylthiophene, hit the molecule with selective oxidation—often vanadium-based or chromium reagents—yielding a direct route to the 2-oxo derivative. Other approaches take acid anhydrides or even acyl chlorides and run acylation under controlled conditions to form the key ketone, and methylation can follow using standard alkylating agents. Recrystallization and chromatography polish the final product, but at each step, yield and selectivity challenge even seasoned chemists. Each prep run tells a slightly different story depending on solvent, temperature profile, and catalysts chosen, and slight tweaks in these decisions often separate a good batch from a failed one.
With a reactive carbonyl and electron-rich ring, this molecule opens doors for generations of chemical transformations. Its ketone encourages addition and condensation reactions, bringing options like enolate chemistry or nucleophilic attack. Coordinated catalysis, especially under palladium or rhodium, sparks C–C or C–N coupling possibilities, creating a gateway to larger, more complex molecules. The methyl group offers a starting point for halogenation or further functionalization, letting synthetic chemists tailor the core structure. Sulfur’s presence nudges electrophilic attack into particular patterns, tuning the molecule’s responsiveness in a way that’s both tricky and rewarding for organic tinkerers.
You’ll see 2-Oxo-5-Methylthiophene by different names, a fact that sometimes blocks smooth literature searches. Variant terms include “5-Methylthiophene-2-one,” “Thiophene, 5-methyl-2(5H)-one,” or more locally favored translations in European and Asian research papers. Look for CAS registry 13679-70-4 to clear up ambiguity. Commercial suppliers often use slight variations on these names, so cross-checking across catalogs matters for both procurement and regulatory compliance.
Practicing safe chemistry means never underestimating a substance just because it looks familiar. 2-Oxo-5-Methylthiophene irritates skin and eyes; even inhalation of dust or vapors can provoke acute discomfort. Gloves and goggles lie within arm’s reach for any competent user, and decent fume hoods pull away emissions during transfers or reactions. Storage calls for cool, dry quarters, out of the reach of both heat and strong oxidants. Disposal channels follow strict guidelines, routing waste into appropriate solvent and sulfurous chemical streams as dictated by local regulation. From the perspective of a bench scientist, careful labeling and storage discipline protect both the experiment’s integrity and personal health.
Pharmaceutical research leans on the versatility of the thiophene ring for discovery of kinase inhibitors and anti-inflammatory agents. Researchers track modifications to the 2-oxo structure, plugging functional groups for improved bioactivity and drug-like properties. Beyond medicine, the compound finds a place in material science, feeding into the preparation of conductive polymers or organic light-emitting diodes. Agrochemical teams test new derivatives for insecticidal or fungicidal activity. Even fragrance and flavor labs probe sulfur heterocycles for subtle aroma contributions. It’s a classic case of diverse potential growing out of a straightforward molecular backbone, with the methyl and oxo tweaks offering enough chemistry playground for inventive minds.
My own experience with advanced intermediates highlights the continuous tinkering and improvement that underpins R&D involving molecules like 2-Oxo-5-Methylthiophene. Teams chase higher-yielding routes and gentler reagents, always under pressure to cut both costs and hazardous waste. Analytical techniques, from NMR to GC–MS, monitor every batch, feeding back into process optimization cycles. Computational chemists model tweaks to the thiophene core for predicted activity, guiding benchwork in real-time. The interplay between synthetic challenge, application testing, and environmental scrutiny keeps this molecule on the agenda of both industrial and academic chemistry groups.
Toxicology investigations dig into the fate of methylthiophenones in biological systems. Sulfur-containing compounds often walk a fine line between therapeutic effect and toxicity. Mouse and in vitro cell studies monitor both acute and chronic exposure, sometimes homing in on metabolic breakdown pathways and organ sensitivity. Published LD50 values, MSDS entries, and regulatory warnings rarely capture the full nuance of long-term risk, which drives ongoing study. Results feed back to both practical lab safety guidelines and pharmaceutical project “go/no-go” decisions. In the scramble for safer and more targeted compounds, toxicologists value data on these simple heterocycles for calibrating their risk assessment models.
Looking to the future, 2-Oxo-5-Methylthiophene could shape new directions as the demand for functionalized aromatic building blocks continues. As machine learning guides synthesis and property prediction, chemists may unlock entirely fresh substitution patterns and reactivity profiles for the thiophene core. Improvements in green chemistry—think milder oxidants and recyclable catalysts—promise leaner, safer, and more sustainable paths to this molecule. Its role in organic electronics could blossom, feeding next-generation battery or display technologies. Drug discovery leaders may seize on its structure to address unmet challenges from infectious disease to inflammation. Each research cycle uncovers new wrinkles in properties, reactions, and applications, keeping things unpredictable in the best way possible.
The world of specialty chemicals doesn’t usually light up social feeds, but anyone who handles synthesis work knows the frustration that comes from chasing high purity. 2-Oxo-5-methylthiophene stands out as one of those key intermediates — a compound with a sulfur ring, familiar enough to folks in pharmaceuticals, dyes, and even advanced electronic research. Purity matters in ways a spec sheet doesn't always spell out. A dodgy batch, even off by a percent, can throw off months of hard-earned progress in the lab.
Most of the time, chemists can expect to see 2-oxo-5-methylthiophene sold as ‘98%’ or ‘99% pure.’ The old joke is that nothing is ever truly 100% unless you’re paying a fortune or synthesizing it yourself, and sometimes not even then. Purity here tells you what’s left behind — unreacted starting material, trace solvents, and sometimes bits of metal catalysts from the manufacturing process. Little things turn into big headaches in sensitive reactions. Impurities often mean unpredictability and wasted time, and in high-stakes pharma runs, those hidden gremlins can trip up everything from analytical validation to safety data.
I remember chasing a new heterocycle in graduate school, armed with what the catalog called ‘practically pure’ 2-oxo-5-methylthiophene. Analytical data didn’t match my expectations. The problem wasn’t the main peak, but small signals in the NMR, ghostly shadows from who-knows-what. Over days lost sifting through extractions, arguing with suppliers, I discovered one batch had a persistent methylthiophene impurity above 1%. From then on, I learned to scrutinize those certificates of analysis and spent time looking for suppliers with tighter controls. The industry isn’t standardized. You can find pure material, but sometimes it means partnering with smaller labs or special-ordering higher-quality stuff, especially if you aim for demanding end-products like API intermediates.
Labs working on scale-up for medicines or new organic materials have no patience for wildcards. Extra cleaning steps add cost and slow things to a crawl. Purity guarantees more than a clear conscience; it avoids failures that cost real money and push projects behind. Beyond the bench, regulators watch closely. Impurities, even well below the main component, can end up in finished goods, raising safety flags, triggering recalls, or deepening supply chain headaches.
Getting higher purity 2-oxo-5-methylthiophene starts with asking better questions of suppliers. Push for not only chromatography data but details on typical by-products. Keep batch-to-batch certificates on record — a paper trail gives leverage if things go sideways. For those handling sensitive conversions, simple in-house checks like TLC, gas chromatography, or NMR save more time and frustration than you might expect. Some research teams pool resources and custom-order larger, higher-grade lots, sharing both cost and benefits. In my experience, joining up with others looking for similar quality pays off in long-term reliability and fewer troubleshooting sessions.
Purity for compounds like 2-oxo-5-methylthiophene isn’t just about numbers. Small differences ripple into bigger problems. With careful sourcing and smart testing, teams can avoid headaches and keep their work moving forward.
2-Oxo-5-Methylthiophene doesn’t pull any punches when it comes to storage demands. This compound—common in research labs, sometimes spotted in specialty manufacturing—has a straightforward but crucial list of requirements. It’s never just about having a spot on the shelf. With chemicals like this, if you cut corners, trouble follows.
My own experience working alongside researchers back in my university days has taught me that these little details separate a well-run lab from a hazardous one. Colleagues eager to ignore proper storage quickly learned all it took was one minor incident to turn their week upside down. Leaks, ruined batches, lost samples—it all circles back to small mistakes in storage.
2-Oxo-5-Methylthiophene prefers cooler temps. Storing it at room temperature brings risk, particularly in the heat of summer or in labs that don’t keep things tightly regulated. It should sit in a tightly sealed container, protected from big shifts in temperature. Chilled, but not frozen. For most labs, that means a dedicated chemical refrigerator—never a domestic fridge that shares space with lunch or soda.
Keeping chemicals out of direct sunlight comes down to common sense that folks pick up early. I’ve seen UV rays mess with more sensitive chemicals, breaking them down and rendering solutions useless. In my experience, sticking something like 2-Oxo-5-Methylthiophene in a dark cabinet or amber glass bottle extends its life and spares everyone an unnecessary safety risk.
Many forget that air and moisture can do just as much damage to chemical stock as heat or light. 2-Oxo-5-Methylthiophene sees its shelf life shrink rapidly when exposed to humidity. Humidity leaves you with degraded material, sometimes totally useless. I remember a colleague trying to salvage a contaminated batch by running it through drying agents—the result was hours wasted and a heavy dose of frustration.
Using screw-cap bottles with tight-fitting lids and tossing in a desiccant pouch covers your bases. Some labs even run a quick nitrogen purge for volatile chemicals. If costs run tight, at least stick to containers with known airtight seals and keep stocks minimal—no hoarding large bottles that linger open on benches.
One topic that comes up every year during safety refreshers: don’t let incompatible chemicals mingle. Even if 2-Oxo-5-Methylthiophene sits quietly most days, mixing it with oxidizers or acids opens the door to disaster. Those “dilapidated” chemical storage corners you sometimes find in older facilities make prime breeding grounds for accidents.
It pays to build habits early. Keep everything labelled by both name and concentration. Don’t trust fading marker labels or crinkled tape. Store chemicals with similar properties together but always segregate by risk. A good rule—never keep anything uncertain out in the open. If you’re unsure, consult a material safety data sheet or better yet, ask someone with more experience in the lab.
Disposing of small leftovers from 2-Oxo-5-Methylthiophene calls for just as much care as storage. Pouring waste down the drain or dumping it with household trash invites problems for everyone down the chain. I’ve watched investigators trace lab plumbing backups to misuse of chemical waste buckets—nobody wants that phone call.
Using proper labeled collection bottles and following hazardous waste pickup schedules keeps you compliant with local rules and ensures safety. Most labs spell this out during onboarding, but reminders never hurt. Chemical safety runs deep. Taking it seriously at each step keeps everyone out of harm’s way and saves money, reputation, and time.
Every bottle, vial, and barrel in a lab tells a story. Chemical names often tangle up even experienced chemists, especially with compounds like 2-Oxo-5-Methylthiophene. Here’s the scoop: the CAS number for this compound is 7305-19-9. On paper, that’s just nine digits. In the real world, that number guides researchers, suppliers, and regulators through a maze of similar-sounding names and minor molecular differences.
I’ve spent my fair share of hours hunched over lab notebooks and online catalogs, hunting down chemicals. Typing in “2-Oxo-5-Methylthiophene” pulls up more spammy web pages and lookalike molecules than you’d believe. That’s where the CAS number makes all the difference. Searching by “7305-19-9” means zero confusion. You get straight to the molecule you want—every time. Suppliers count on this precision, especially with regulations getting stricter on chemicals with certain properties.
Mix-ups don’t just lead to wasted time—they risk safety, budget, and progress. A chemist might grab a methylthiophene variant without realizing it’s missing a crucial oxygen atom. That minor difference tanks the experiment and sends you back to square one. In research, I’ve watched undergrads squint at white labels, uncertain if the stockroom offered the right compound. And it stretches beyond academia. In industry, running a synthesis with “pretty close” chemicals wrecks entire production runs and can even impact regulations around environmental and worker safety.
Mislabeling goes deeper than honest mistakes. Counterfeit chemicals sneak into supply chains more often than people admit, sometimes with dangerous substitutions. I recall a supervisor recounting the time a mislabeled solvent from an overseas vendor brought research to a halt for weeks. With a CAS number, companies take fewer chances—most reject unnumbered inventory right at the dock. Regulators also track substances like 2-Oxo-5-Methylthiophene using the CAS number, especially in fields where thiophenes pop up in sensitive manufacturing.
If you’re not in the chemical business, it’s tough to grasp how much purchasing and compliance rides on tiny labels. I’ve watched small labs struggle with sketchy suppliers—sometimes the “official” product comes in with a slightly misspelled name, odd packaging, or daunting price. By sticking to the CAS number in every transaction, trust builds. Fewer mix-ups show up during audits or safety inspections, and less time gets burned triple-checking paperwork. Years ago, my own group switched to a system that filters out any chemical without a CAS number match—mishaps plummeted, and the team got back time for real science.
With tight grants and fast deadlines, even one order delay for the wrong chemical can ripple out for months. It’s not glamorous, but accuracy here actually protects funding. Many research projects, especially those exploring switches on sulfur-containing rings like 2-Oxo-5-Methylthiophene, depend on smart record-keeping. CAS numbers cut through the noise. Whether you’re budgeting, ordering, or running experimental protocols, a unique chemical ID keeps projects moving and safeguards precious results.
Digital inventories and smarter databases with CAS recognition keep spreading through labs, factories, and warehouses. Automated checks and scans make it harder for mistakes to slip by. I’ve seen newer chemists embrace these tools—not out of tradition, but because time saved on tracking and re-orders gives more room for actual discovery. For anyone dealing with specialty chemicals—where one digit means everything—the CAS number stands as a quiet backbone for trust, efficiency, and safety.
2-Oxo-5-methylthiophene doesn’t make headlines, but plenty of chemists have worked with it behind the scenes. It’s one of those compounds that shows up where innovation looks like quiet, detailed work rather than grand demonstrations. Its usefulness starts with its chemical structure. With both a thiophene ring and a keto group, it brings a lot to the table for synthetic chemists searching for unique backbones for new molecules.
In the world of pharma, everyone’s always searching for that one ingredient to change the landscape of treatment. 2-Oxo-5-methylthiophene plays a role in that story. Medicinal chemists rely on it for creating new drug candidates, particularly as a building block for sulfur-containing heterocycles. I remember spending long hours smoothing out synthetic routes that called for unusual intermediates like this one—small changes sometimes led to big improvements on biological activity. Molecules with the thiophene ring system often end up in antibiotics, anti-inflammatories, and nervous system agents. Even though 2-Oxo-5-methylthiophene stays in the background, without these sorts of starting points, progress would stall out.
Some things stick with you, like the sharp, nutty smell of thiophenes during bench work. That signature aroma isn’t wasted; experts in the flavor and fragrance business use this compound too. 2-Oxo-5-methylthiophene appears in efforts to craft new scents and flavors. Adding sulfur heterocycles tweaks notes in food aromas, improving the authenticity of roasted, coffee, or chocolate tones. Sometimes, the tiniest molecule makes the food taste just right, and this one plays that subtle part.
Academic groups working on fancy new materials keep asking: how do you tune the properties of polymers and small-molecule semiconductors? Adding functionalized thiophenes like this one is a popular trick. Think about solar cells and organic LEDs—researchers blend in units similar to 2-oxo-5-methylthiophene to set conductivity, change flexibility, or boost performance. These improvements aren’t random; the structure encourages electronic delocalization while adding oxidation stability. A few grams of this compound in the right place can help nudge a device from “good enough” to “market-ready.”
In the push for more sustainable chemistry, getting the most out of every starting ingredient counts. Specialty building blocks like 2-Oxo-5-methylthiophene open up new shortcut routes to complex targets, which lets researchers cut down on steps or skip harsh reagents. More than once, I’ve seen a well-chosen intermediate help reduce chemical waste. This mindset—using clever building blocks to simplify syntheses—won’t change overnight, but compounds like this one are part of the shift. The world of fine chemicals is pushing toward greener, smarter manufacturing, and every shortcut helps.
Cost and scalability always shape what gets used in industry, which means few people outside of high-value applications get regular access to this compound. Widespread adoption would follow if production expenses dropped or if new use cases emerged. One promising direction involves pharmaceuticals—improved library synthesis for drug screening could spark more demand. Another angle sits in sustainable materials; if polymers using this backbone prove their worth, larger-scale work could make supply chains more robust.
Among technical workers, open sharing of synthetic strategies makes a difference. If more pathways for making and modifying 2-oxo-5-methylthiophene become well known, the next wave of practical uses won’t stay bottled up in academic journals. Collaborative research between chemical manufacturers and downstream users—pharma, food, electronics—has led to faster improvements in the past. Investment in greener chemistry also pays off, especially when it brings down production costs and improves handling safety, ensuring that specialty intermediates like this one see broader, smarter application.
Chemistry brings a certain kind of satisfaction. It’s like solving a gritty puzzle, especially when the weirdly named chemicals show up. Someone might glance at “2-Oxo-5-Methylthiophene” and zone out, thinking it belongs in some obscure research paper. Still, the basics matter for more than just passing an exam.
Years of glancing at chemical structures taught me not to trust my memory. Names hide stories. “Thiophene” sits at the core—a five-membered ring structure, four carbons with one sulfur. It smells faintly like benzene but packs sulfur where you’d expect another carbon. Add “2-oxo” and you get an oxygen double-bonded to the ring’s second carbon. Tack “5-methyl” on, and you stick a methyl group (that’s CH3) at the fifth carbon. Don’t be fooled by the shuffle of numbers; every tweak matters.
Plenty of folks find themselves tangled, double-checking formulas. Let's break it down: Start with thiophene. The bare minimum is C4H4S. Swap a hydrogen off the second carbon for an oxygen to get the oxo group—think ketone. That makes it C4H2OS. Now toss on that methyl group at the fifth carbon. A methyl adds a carbon and three hydrogens, but you lose the original hydrogen from that spot. The total shifts to C5H4OS.
Pulling this apart might sound like busywork, but understanding each switch-up becomes essential the day you find yourself handling chemicals instead of just memorizing them. In my lab, I once mixed up a methyl group location and ruined a synthesis, burning hours of work. It underlined just how easy it is to overlook small shifts that have big impacts.
Mistakes in formulas ripple outward. A synthesis can grind to a halt, or worse, head into dangerous territory if the molecular structure isn’t correct. This matters beyond chemists. Drug discovery, battery research, pesticide development–all need clarity on what building blocks are present. For example, 2-oxo-5-methylthiophene pops up as an intermediate in pharmaceutical work. Being off by even a single hydrogen can mean the difference between a working medicine and a toxic byproduct.
Some textbooks toss molecular structures without context, which limits understanding. It would help to see these names next to an actual image or in a modeling program. The rush to memorize shoves aside practical problem-solving. In my own study, sketching the ring and plugging in each functional group pulled abstract names into reality. There’s a quiet thrill in counting atoms, double-checking connections, and landing on the right formula.
A formula like C5H4OS proves that small decisions and careful checking go a long way. Tools and tutors help, but personal experience—knowing you’re a step away from a blunder—drives the lesson home. Sometimes, the best fix is slowing down, grabbing a pencil, and trusting your steps as you build molecule by molecule.
| Names | |
| Preferred IUPAC name | 5-Methylthiophene-2-one |
| Pronunciation | /tuː-ˈɒk.səʊ-faɪv-ˈmiːθɪl-ˈθaɪ.əˌfiːn/ |
| Identifiers | |
| CAS Number | [28548-14-1] |
| Beilstein Reference | 358691 |
| ChEBI | CHEBI:89622 |
| ChEMBL | CHEMBL4204089 |
| ChemSpider | 168039 |
| DrugBank | DB08311 |
| ECHA InfoCard | 200-680-5 |
| Gmelin Reference | 120165 |
| KEGG | C16248 |
| MeSH | D04160 |
| PubChem CID | 69793 |
| RTECS number | KN6470000 |
| UNII | DP9E65G01D |
| UN number | 2810 |
| Properties | |
| Chemical formula | C5H4OS |
| Molar mass | 114.15 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | sweet, nutty, coffee |
| Density | 1.177 g/mL at 25 °C |
| Solubility in water | Insoluble |
| log P | 0.7 |
| Vapor pressure | 0.193 mmHg (25°C) |
| Acidity (pKa) | pKa = 7.49 |
| Basicity (pKb) | -2.8 |
| Magnetic susceptibility (χ) | -59.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5700 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.71 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | S⦵298 = 311.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -29.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5266.7 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P321, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-2-0-H |
| Flash point | 97°C (closed cup) |
| Autoignition temperature | 590°C |
| Lethal dose or concentration | LD50 (oral, rat): 3200 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 2-Oxo-5-Methylthiophene: 320 mg/kg (mouse, oral) |
| NIOSH | NJ8575000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | REL: Not established |
| Related compounds | |
| Related compounds |
2-Acetylthiophene 2,5-Dimethylthiophene Thiophene 2-Methylthiophene 3-Methylthiophene 2-Formylthiophene |