Chemists started looking at thiophene derivatives long before the digital age took off. Interest in 2-Oxo-5-Benzoylthiophene started picking up pace during the second half of the twentieth century, as advances in heterocyclic chemistry made it possible to isolate and modify sulfur-containing rings with high yield and precision. As a student in a modest university lab, I watched mentors synthesize new thiophene compounds, always hoping for a molecule that would open doors in material science or medicine. Back then, getting a pure batch of 2-Oxo-5-Benzoylthiophene called for patience, trial and error, and more than a few spillage stories. Over several decades, researchers refined steps for attaching a benzoyl group to the thiophene ring, motivated by the unusual reactivity this compound displayed. Today, access to high-purity samples no longer feels out of reach, but the story of its discovery speaks volumes about the energy and creativity that keep organic chemistry so alive.
2-Oxo-5-Benzoylthiophene stands out for the way its unique structure brings together a thiophene ring with both a benzoyl group and a ketone function. This fusion leads to a building block many labs prize, whether the goal is to tweak pharmaceuticals, engineer advanced polymers, or investigate new catalysts. As someone often buried in catalogs and paperwork, I’ve noticed suppliers tend to offer the compound as a pale yellow crystalline powder, clearly labeling for research use only. The price per gram sometimes feels steep, but researchers hunger for its versatile reactivity, and demand stays strong.
The physical fingerprint of 2-Oxo-5-Benzoylthiophene holds steady: crystalline, melting around 117–119°C, often giving a faint but sharp odor typical of benzoyl derivatives. Solubility in organic solvents—think dichloromethane, ethanol, or acetone—makes it practical for synthetic work across small and large scales. On the chemical front, the conjugated system spanning the thiophene and benzoyl moieties brings a degree of stability that resists easy breakdown, even under moderate heat. Yet this same system can unlock a world of transformations at the right hands, drawing on both electrophilic and nucleophilic partners. From the bench, the fine line between inertness and reactivity becomes a playground for those seeking to build new structures or test reaction mechanisms.
Every reputable bottle of 2-Oxo-5-Benzoylthiophene comes with detailed specs: purity (often above 98% high-performance liquid chromatography), batch number, storage guidance (cool, dry, light-protected), hazard identification, and molecular weight (240.27 g/mol). Certificates of analysis go beyond box-ticking—they’re the foundation of repeatable, defensible research. I’ve learned that rushing this step can compromise whole projects. Container labels usually print the CAS registry number (72930-59-5) and offer pictograms for health risks, reminding users to glove up and avoid every temptation to treat these powders lightly.
Laboratories synthesize 2-Oxo-5-Benzoylthiophene by Friedel-Crafts acylation. The process begins with a thiophene substrate, which reacts with benzoyl chloride under the watchful presence of a Lewis acid catalyst—commonly aluminum chloride—a method first described decades ago and still in common use. Witnessing this reaction never ceases to teach humility; it produces a mixture of products, and careful workup—solvent extraction, column chromatography, iterative recrystallizations—yields the pure target compound. Some groups now favor greener strategies, swapping toxic chlorides for milder agents or ionic liquid-based systems. These tweaks cut down on hazardous waste and keep workspaces safer, though the old school methods persist for the chemists clutching their handwritten lab notebooks.
The structure of 2-Oxo-5-Benzoylthiophene offers an enticing platform for further chemistry. As someone who spent long days at the hood, I’ve watched this compound serve as both electrophile and nucleophile, giving rise to all manner of transformations. Reductions transform the ketone to alcohol; nucleophilic additions on the benzoyl group allow for creation of imines or hydrazones, depending on the conditions. Common derivatizations harness its conjugated system to build new aromatic rings, cross-couple with boronic acids, or even anchor metal centers for catalysis. Reactions with amines, alkenes, or active methylene compounds can tailor-make custom scaffolds for pharma or material science applications. The possibilities remind one why simple structures remain the cornerstone of advanced synthesis: there are always more ways to flip the molecular script.
2-Oxo-5-Benzoylthiophene appears under a handful of synonyms in the literature and in supplier catalogs. The most common alternate names you’ll see include 5-Benzoyl-2-thiophenone, sometimes shortened to Benzoylthiophenone for practical listing. Some chemists refer to it by its registry number or shorthand for longer IUPAC descriptors. As much as the names might shift, those who know their organosulfur chemistry recognize the structure on sight.
In the lab, respect for hazardous materials runs deep, and 2-Oxo-5-Benzoylthiophene demands no less. While acute toxicity doesn’t top the highest risk categories, the compound can irritate the skin, eyes, or respiratory tract. Handling it drags up memories of early missteps—spilled aliquots, missed glove swaps, and accidental eye rubs led to plenty of lessons learned. Local exhaust ventilation, face shields, and double nitrile gloves often enter the scene. Detailed safety data sheets back up these routines, outlining what to do in case of spills and exposure, and tracking waste through proper disposal channels. Ignoring these steps rarely ends well.
Research groups and industries use 2-Oxo-5-Benzoylthiophene as a foundation for pharmaceutical intermediate synthesis, piquing the curiosity of medicinal chemists targeting molecules for antimicrobial and anti-inflammatory roles. In other quarters, its photoactive skeleton draws material scientists in, who blend it into polymers, optoelectronic devices, or as probes for solvent polarity. I’ve seen more than one team screen new derivatives for light absorption or electrical performance, searching for that extra bump in efficiency. Its modest toxicity and manageable reactivity open the door for instructional lab exercises, giving students a taste of advanced synthesis without excessive risk. The compound also ranks as a key intermediate for natural product analogues, tying modern labs to centuries of exploratory organic chemistry.
The growth of 2-Oxo-5-Benzoylthiophene research traces back to persistent questions about how simple molecules can seed entire fields. In my own experience, every time a group accessed a new analytic technique—better NMR, advanced crystallography, sharper mass spec—they revisited “classic” compounds like this one, revealing new patterns of reactivity or unexplored side products. Modern projects look for eco-friendly substitutes for synthesis steps, scrutinize kinetic behaviors in real time, and scan the molecule against libraries of biological targets. Funding agencies press for applications in green chemistry and medical innovation, prompting researchers to squeeze every last insight out of a well-characterized but still enigmatic set of reactions.
Toxicological evaluations for 2-Oxo-5-Benzoylthiophene still rest on a small but growing body of data. Acute exposures in rodents suggest low-to-moderate oral toxicity, but the real focus remains on chronic effects and pathways of metabolism, especially with long-term contact. In-journal studies compare its structure to related benzoyl-thiophene compounds implicated in mild mutagenicity. Lab animals exposed to high doses displayed liver stress and selective enzyme changes—findings that put teeth behind regular wearing of personal protective equipment. Environmental studies flag its slow degradation in water systems and point to the need for careful waste management in manufacturing settings. I’ve shared coffee with toxicologists who weigh the compound’s promise against these uncertainties, always eager for more reliable exposure models and biomarker tracking.
The path ahead for 2-Oxo-5-Benzoylthiophene combines old wisdom with new ambition. Better synthesis and purification techniques, including flow chemistry and machine-driven reaction optimization, promise time and resource savings. Medicinal chemists hope to wring out every last possibility as a scaffold for new drugs, especially antimicrobial or anti-cancer candidates. Renewable materials researchers keep an eye on its potential for next-generation solar films or biocompatible polymers. Calls for sustainability will likely drive safer, lower-waste production protocols. In my own lab, I see growing interest from students who want to push the boundaries, eager to turn well-trodden ground into entirely new chemistry. Every project offers a reminder that molecules like this, first isolated with glassware and patience, still have a long story ahead.
Few folks outside research circles get excited by something like 2-Oxo-5-Benzoylthiophene. That name rolls off nobody’s tongue at a family BBQ. Still, this little molecule pulls its weight in real-world ways. The first time I came across it, a friend studying pharmaceuticals mentioned it over coffee as part of her project on anti-inflammatory drugs. Back then, it sounded like just another research chemical, but a deeper look told a more interesting story.
2-Oxo-5-Benzoylthiophene lands itself in pharmaceutical labs thanks to its unusual structure. Scientists often use thiophene rings in drug design because they help tweak how medicines interact with the body. Adding a benzoyl group and a keto group to the ring changes its reactivity. Companies make good use of it as a building block when developing new medicines, especially those meant to target inflammation or pain. A team in India recently shared results where a compound based on this molecule showed promise against certain bacterial infections.
The world’s always searching for new antibiotics and anti-inflammatory agents. Overuse of old drugs leads to resistant bugs, and plain old ibuprofen doesn’t always get the job done. Compounds built from 2-Oxo-5-Benzoylthiophene open more paths, giving researchers unusual skeletons to work with when the usual chemical shapes stop working. Just last year, a PhD student at a German university found that tweaking its side groups affected how it blocked inflammation in animal trials.
Companies that make custom chemicals for research list this compound on their catalogs. From what chemists tell me, it serves as a key middle step while stitching together more complex drug molecules. If you spend time paging through chemical databases, its name keeps popping up in multiple patent applications for painkillers, anti-fungal creams, and cancer research. For smaller research outfits, being able to order a bottle of something like this instead of starting synthesis from scratch saves time, money, and a little gray hair.
No one grabs headlines with stories about starting materials. Still, without access to things like 2-Oxo-5-Benzoylthiophene, a lot of medical progress would crawl. Sourcing or making specialty chemicals often means wading through safety regulations, rising raw material costs, and sometimes even factory shortages. Global disruptions over the past few years have made getting hold of these chemicals trickier, sometimes slowing down projects meant to improve human health.
The world’s got no shortage of tough medical problems, and the solutions often start in small bottles labeled with unpronounceable chemical names. Small suppliers, universities, and pharmaceutical companies need to keep lines of communication open. Sharing production methods and pooling resources can dodge bottlenecks and fuel new breakthroughs. In my own visits to chemistry labs, I’ve seen how a bit of open sharing pushed a stalled research track forward that year. Developers benefit when labs don’t have to fight over who gets a rare chemical — everybody wins if new medicines hit the market sooner.
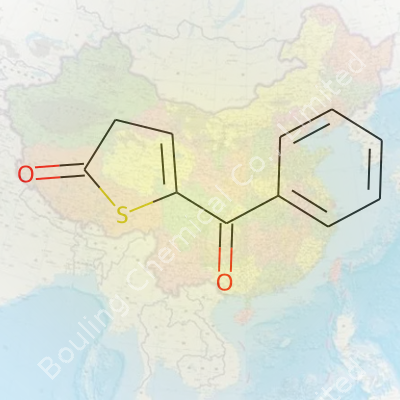
Chemistry can feel like a tangled forest, full of names and numbers that seem impossible to remember. One name that catches the eye is 2-Oxo-5-Benzoylthiophene. Sounds complicated, but this compound isn’t as mysterious as it may sound. The chemical formula—C13H8O2S—lays out a simple map: thirteen carbons, eight hydrogens, two oxygens, one sulfur. If you toss these building blocks on a scale, you get a molecular weight of 228.27 g/mol. That’s the kind of figure you see scribbled in old lab notebooks, the sort that sticks in your head if you’ve spent any real time behind a chemistry bench.
This particular combo of atoms packs more punch than its size lets on. Thienyl-based compounds show up across pharmaceutical projects and dyes. Having a benzoyl group slapped onto a thiophene ring, topped off by a well-placed keto group, really changes what the molecule can do. These details matter for folks making decisions about drug design or those who tinker with advanced materials.
Chemists don’t spend time memorizing formulas just for kicks. That formula, C13H8O2S, tells you almost instantly that there’s aromaticity in the mix—rings that add stability and open doors to interesting chemical behavior. Experience with sulfur-containing rings shows just how small tweaks in structure affect how a molecule interacts with enzymes or light. From my time patching together synthetic routes in grad school, a small sulfur atom often means much bigger surprises during a reaction.
If you jump into pharmaceutical chemistry, that molecular weight—228.27 g/mol—feels like a sweet spot. It isn’t so heavy that it drags through a biological membrane like an anchor. It isn’t so light that enzymes scarf it up without a fight. This balance sits at the crossroads where medicinal chemists look for the next lead compound.
With a backbone like this, there’s always risk: unexpected side reactions, tricky purifications, or toxicity hiding behind the benzoyl handle. Folks trying to scale up synthesis in industry can find themselves elbow-deep in chromatography, looking for better purification tricks or green solvents that don’t threaten their health or the planet. The academic labs I trained in would run through gallons of ethyl acetate and hexanes—two solvents that spell trouble for both workers and the world. Leaning on newer purification tools, greener solvents, and digital planning can slice down that waste and make scaling this molecule a bit less daunting.
The formula and molecular weight sit at the top of every data sheet for good reason. Quick access means fewer mistakes and faster troubleshooting, especially when working under pressure. Research groups would benefit from tools that spit out this information in a heartbeat, taking away the grunt work and letting folks focus on the tough stuff—creating new medicines, brighter materials, and more effective catalysts.
A compound like 2-Oxo-5-Benzoylthiophene looks simple on paper, but it opens up conversations about sustainable chemistry, smarter research, and taking the work from a test tube to the real world. Those small details in its formula and weight set the stage for everything that follows.
Anyone who's worked with chemicals knows sloppy storage can cost plenty, from lost materials to ruined experiments or even safety incidents. 2-Oxo-5-Benzoylthiophene shows up in research labs all over, mostly because it acts as a valuable building block for all sorts of organic syntheses. It’s no household name, but for chemists, it’s a familiar sight on the reagent shelf. Experience has taught me that you can’t treat every powder or liquid the same way. Mistakes add up, especially with compounds carrying sensitive bonds or the tendency to break down.
Let’s cut to the chase—a bottle of 2-Oxo-5-Benzoylthiophene belongs in a climate of steady conditions. Humidity tends to be the enemy. Moisture can creep through even well-sealed containers, clumping powders or triggering slow changes, which spells trouble down the road. That's why a desiccator makes such a difference. Toss in some fresh desiccant packs, and you'll stay ahead of moisture sneaking in.
Now about the heat—high temperatures can turn a stable compound into a headache pretty quick. Top shelves catch stray warmth from overhead lights; avoid them. I’ve always aimed low, stashing sensitive chemicals near the bottom of the storage cabinet where it stays cooler. A solid temperature target sits between 2℃ and 8℃. This range lines up with what most laboratory fridges provide, but double-check the label. Some compounds settle for shelf storage at room temperature, others fare better in the fridge.
Sunlight or even harsh lab lighting brings an extra risk. UV rays pack more punch than people think, breaking bonds and causing color changes, sometimes in just days. Amber glass bottles do a solid job at blocking most of this, but don’t just trust the glass alone. Tuck bottles away in cabinets with doors or opaque bins. That simple move spares reagents from slow breakdown nobody wants.
Labels matter more than people admit. Over the years, I’ve come across too many mystery bottles, their scribbled notes long faded, and the contents anybody’s guess. Permanent labels with clear names, concentration, and opening dates cut straight through that confusion. Sorting bottles by hazard level and usage stops mix-ups and saves time during busy experiments.
A loose lid adds risk. Vapors escape, moisture enters, spills happen. Regularly tightening caps and inspecting for cracks or residue can make the difference. I never forget the day a friend discovered a corroded lid, leaking pungent fumes into an otherwise tidy cabinet. The cleanup took hours, and the wasted material cost plenty. Choosing bottles with tight, chemical-resistant caps pays off. Swapping out compromised containers comes easier than dealing with lost product or damaged shelves later.
The best labs run smoothly because nobody gets complacent about how chemicals sit on the shelf. Lab accidents rarely come from big, obvious mistakes—they sneak in while people overlook the basics. Keeping 2-Oxo-5-Benzoylthiophene safe demands a cool, dry, and dark space, a tough, well-labeled container, and an end to careless habits. Taking these simple steps gives everyone peace of mind and proves small choices matter.
Anyone who has spent time around a chemistry lab knows the hidden risks behind new compounds. 2-Oxo-5-Benzoylthiophene is the kind of chemical that doesn’t pop up in splashy headlines, but it brings a set of challenges worth talking about. You don’t often find it in starter lab kits or undergraduate organic chemistry classrooms. Most people dealing with it are lab technicians, researchers, or chemical engineers.
Some compounds walk into a room with reputation. This one has an odd pedigree: not common, but used for certain pharmaceutical syntheses, sometimes as an intermediate building block. On paper, it doesn’t scream danger like hydrofluoric acid or phosgene. That doesn’t mean you can toss it around. A scientific background teaches a healthy respect for any synthetic chemical that isn’t listed as food-safe or broadly tested in humans.
Look at its structure — a thiophene ring, benzoyl group, and a reactive oxo moiety. Whenever sulfur is in play, eyes start to dart to the safety sheet. Pure thiophenes can carry toxicity. Conjugated carbonyl groups have shown skin irritation or sensitization in close relatives. Literature isn’t overflowing with animal toxicity studies, so this isn’t a situation of “no news, good news.” The absence of clear hazard labels doesn’t guarantee smooth sailing.
Many years in active chemical labs have drilled in an instinct: approach each new bottle like it just arrived from a mystery auction. Gloves, safety goggles, fume hood—these are not suggestions. They’re survival gear. I’ve seen researchers toss caution aside only to regret skipping basic personal protection. You never know what old glassware held before or how finely powdered a solid can get when poured on a humid day.
Safety Data Sheets (SDS) for 2-Oxo-5-Benzoylthiophene often lean on “treat as harmful if inhaled, swallowed, or absorbed through the skin.” This guidance covers a lot: wear protective gloves, eye protection, maybe a lab coat or apron. Don’t sniff the bottle to “see what it smells like.” Avoid getting residues on work surfaces, and keep open flames far from the bench. Just because the SDS lacks boldface warnings like “carcinogen” or “mutagen” doesn’t write a blank check for carelessness.
Waste streams for oddball chemicals often become an afterthought. The real headache starts at the end of a project, as researchers tally up what’s left. Throwing this compound in the trash or sink could lead to legal problems and cost the lab money. Corrosive materials, organosulfur compounds, and unknown by-products can react unpredictably. It’s better to collect unused material in clearly labeled containers and hand it off to a professional disposal service. Rules can differ by country, but basic caution saves everyone stress.
A smart approach goes further than simply avoiding accidents. Posting printed chemical profiles on storage shelves, doing quick chemical inventories, and keeping well-stocked emergency showers all help. New researchers and students learn to respect compounds like 2-Oxo-5-Benzoylthiophene not by reading about someone else’s mishap, but through active training and hands-on supervision.
Chemistry never feels routine. No one gets a pass on vigilance just because a compound seems off the radar. Chemical literacy, reliable safety gear, and strict handling habits turn potentially risky situations into everyday success stories.
Across chemical labs, talk turns quickly to purity grades. With something like 2-Oxo-5-Benzoylthiophene, most chemists I know don’t waste time chasing after mystery grades. The two grades most folks regularly see are research-grade and technical-grade. Research-grade doesn’t just mean it’s ‘good enough’; it often hits 98% or higher on purity. This ensures the results don’t get thrown off by something lurking in the beaker. Think thin-layer chromatography that’s clean, no weird spots when UV hits the plate. If you’re running HPLC, the peaks won’t get chewed up by rogue byproducts. You get that peace of mind—your synthesis is based on what’s actually on the label.
Research-grade gets its reputation from more than purity. Documentation follows, including batch analysis and sometimes elemental analysis. Reputable suppliers cough up the certificates. For graduate students and postdocs publishing papers, this paperwork saves hours of worry later.
Technical grade usually lands between 90% and 96%. In scale-up or pilot runs, technical grade gets picked up first. If you’re working under fume hoods with multi-gram syntheses, paying double for research-grade sometimes just isn’t worth it. Most process chemists keep an eye on cost and don’t mind the extra few percent of impurity, as long as the downstream products aren’t sensitive. You see it most out in pharma intermediates or specialty pigments.
The catch is consistency. One batch might run fine, another may bring unexpected tints or odorous wisps. Technical grade often skips deep analysis. No certificates, just a basic spectrum stapled to an invoice or a line in the catalog saying ‘>92%’.
Occasionally, some labs need even cleaner stuff—think pharmaceutical quality or ‘custom pure’. For example, a group working on new APIs will sometimes shake hands with boutique chemical suppliers, asking for 99.5% or above. Here, every fraction of a percent matters; minor leftovers in starting materials ruin a new compound or show up in regulatory filings. Prices climb, and so does paperwork: full traceability, complete impurity profiles, sometimes even GMP-standard processes to keep the contaminants at bay.
Most buyers don’t see these ultra-pure forms. They’re spoken of at conferences or in contracts, rarely on the open market. The process takes time—sometimes months for a few grams. Still, contract chemists who need an edge to get a patent or to prove a process won’t pollute a batch of life-saving drugs are willing to wait.
Not every country or supplier defines purity the same way. I’ve seen suppliers in one region stamp the same batch ‘analytical grade’, where another calls it ‘technical’. Since naming isn’t standardized, buyers must check COAs and request third-party assays if a project is on the line. I always recommend pulling spot tests; TLC, NMR, or LC-MS can expose big differences. Price sometimes shadows quality, but it never speaks louder than the raw data.
If sourcing for a critical experiment or product launch, reach out to suppliers who show their methods—chromatograms, NMR spectra, even water content checks. Build a relationship. Good communication ensures the batch you get in year one matches the batch in year three. It’s not just about paperwork; real updates and transparency shape reliability, year after year.
| Names | |
| Preferred IUPAC name | 1-Benzoylthiophene-2(5H)-one |
| Pronunciation | /tuː-ˈɒksəʊ-faɪv-bɛnˈzɔɪl-θaɪˈəʊfiːn/ |
| Identifiers | |
| CAS Number | 625-86-5 |
| 3D model (JSmol) | `/opt/local/bin/java -Djava.awt.headless=true -cp JSmol.jar JmolData.jar JmolApplet.jar JmolExport.jar JmolViewer.jar JmolPanel.jar org.jmol.api.JmolViewer "load data '2-Oxo-5-Benzoylthiophene'; write JMOL |
| Beilstein Reference | 346930 |
| ChEBI | CHEBI:91520 |
| ChEMBL | CHEMBL503929 |
| ChemSpider | 161388 |
| DrugBank | DB07910 |
| ECHA InfoCard | 100.041.306 |
| EC Number | 2523-68-8 |
| Gmelin Reference | 107658 |
| KEGG | C18603 |
| MeSH | D016678 |
| PubChem CID | 70937 |
| RTECS number | XN8220000 |
| UNII | SD6Q59I0RH |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | DTXSID4069738 |
| Properties | |
| Chemical formula | C11H8O2S |
| Molar mass | 222.25 g/mol |
| Appearance | Light yellow solid |
| Odor | Aromatic |
| Density | 1.32 g/cm3 |
| Solubility in water | Insoluble |
| log P | 1.98 |
| Vapor pressure | 1.42E-7 mmHg at 25°C |
| Acidity (pKa) | 5.72 |
| Basicity (pKb) | pKb: 10.51 |
| Magnetic susceptibility (χ) | -74.51×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.6420 |
| Viscosity | Viscosity: 1.15 mPa·s (20°C) |
| Dipole moment | 4.44 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 385.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | +2.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -775.6 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P264, P271, P280, P301+P312, P304+P340, P305+P351+P338, P312, P337+P313, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 138°C |
| LD50 (median dose) | LD50 (median dose) of 2-Oxo-5-Benzoylthiophene: "LD50 (oral, rat) > 5000 mg/kg |
| NIOSH | AC9275000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg |
| Related compounds | |
| Related compounds |
2-Acetylthiophene 2-Benzoylthiophene 2-Bromo-5-benzoylthiophene 2-Oxothiophene 5-Benzoylthiophene-2-carboxylic acid |