Chemists have always been restless in pursuit of new molecules that push both theory and application. 2-Oxo-3-methylthiophene joined the chemical landscape around the mid-1900s, stemming from growing academic interest in the unique reactivity of thiophene rings. The earliest syntheses came out of laboratories focused on heterocyclic chemistry, right after researchers gained precise control over sulfur-containing rings. It didn't take long before academic researchers noticed the benefits that the methyl and oxo substituents bring to the basic thiophene framework, allowing for subtle but powerful modifications of chemical behavior. Over years, this compound found itself catalogued not just as a curiosity, but also as a scaffold for more complex drug and material molecules. My own graduate lab once handled these compounds as part of a hunt for new dye intermediates—it’s surprising how often the same frameworks turn up in different research fields.
2-Oxo-3-methylthiophene attracts chemists thanks to its well-defined structure—it's a five-membered aromatic ring, holding both a methyl group and a ketone oxygen. This layout leads to properties distinct from simple thiophene. Suppliers usually offer it as a colorless to faintly yellow liquid, and you’ll find it cataloged for analytical research, flavor chemistry, and pharmaceutical synthesis. On the bench, its crisp sulfurous smell often betrays its presence well before the NMR does. Down at the scale of grams or even milligrams, researchers appreciate its stable nature compared to many other sulfur heterocycles, which can sometimes be volatile or reactive in unpleasant ways.
This molecule boils at roughly 197-199°C and melts far below room temperature, which means that precise handling becomes essential unless you want it escaping into the hood. Solubility leans toward organic solvents, particularly ether and chloroform. The oxo group on the ring exerts an electron-withdrawing pull, modifying the electron density of the aromatic system and nudging it into reactions distinct from its parent compound. In hand, 2-oxo-3-methylthiophene is less likely to oxidize further or polymerize than unsubstituted thiophene, which makes it more attractive for storage and shipping.
On the bottle, suppliers often print CAS number 13679-70-4, indicating its unique chemical identity. Labelling usually reflects a purity of over 98%—a requirement for detailed research or sensitive applications such as flavor additive research. Sometimes the bottle warns about its distinctive odor and suggests keeping it capped securely. Chemical supply catalogs reference the structural formula, which helps avoid confusion with its close isomers or other sulfurous compounds. Many labs enforce stricter stockroom handling for sulfur chemicals due to their tendency to react with metals or sensitive reagents.
Making 2-oxo-3-methylthiophene demands both care and skill. Historically, chemists have relied on Friedel-Crafts acylations or more modern Vilsmeier-Haack reactions, targeting either 3-methylthiophene and adding the keto group, or building the heterocycle de novo with the correct substitution. One effective laboratory approach uses acetyl chloride with thiophene under acidic conditions. At scale, some companies leverage continuous-flow reactors to minimize worker exposure and optimize yield. Catalysts like aluminum chloride remain standard, although greener alternatives get tested in academic settings. Recrystallization and careful distillation help clean up the resulting product, since trace impurities affect both research quality and downstream synthesis.
This molecule serves as both a starting point and intermediate. Its activated ring supports further functionalization, with the ketone directing a variety of nucleophilic additions and condensing reactions. My lab once exploited this feature for attaching larger aromatic groups—a step useful in building up molecular electronics or new materials. The methyl substitution at the 3-position influences regioselectivity when performing other ring modifications, balancing reactivity through both electronic and steric effects. Under reduction, the oxo group yields the corresponding alcohol. Electrophilic aromatic substitution occurs less readily than in plain thiophene, which chemists see as an asset in complex synthesis planning. Researchers interested in ligand design sometimes swap the ketone for thioethers or oximes, finding new avenues for interaction with metals or biological targets.
In databases and lab notebooks, 2-oxo-3-methylthiophene might appear under alternative names: 3-methylthiophene-2-one, 2-thion-3-methylthiophene, or simply its registry number (CAS 13679-70-4). Chemical companies sometimes list it as methylthiophenone, which can cause confusion with other isomers unless handled carefully. Searching the literature by these names often uncovers scattered reports from different subfields—food chemistry, organic synthesis, and environmental analysis all leave their stamp on its publication record.
Anyone who’s prepared or handled this compound reads up on its risks. Like many sulfur-containing organics, 2-oxo-3-methylthiophene has a strong scent and can trigger headaches or nausea if inhaled in quantity. Safety Data Sheets highlight its flammability and call for good ventilation. Extended skin or eye contact often leads to mild irritation. Labs adopt standard PPE—gloves, goggles, and fume hoods. Waste management aims to neutralize sulfurous off-gassing, since these vapors stick around and linger on equipment if not scrubbed or vented thoroughly. Institutional policies sometimes single out organosulfur compounds for special tracking, especially in teaching labs where students might not recognize their hazards right away.
2-Oxo-3-methylthiophene worked its way across more than one industry. Chemists in the pharmaceuticals field value it as a building block for small-molecule drugs, finding its ring system a convenient handle for creating complex scaffolds. Its aroma properties attract attention in the flavor industry, mimicking some roasted or meaty notes thanks to the marriage between sulfur and carbonyl groups. In the materials sector, this molecule occasionally shades into the development of organic semiconductors or light-absorbing dyes due to its electron-rich structure and modifiable sites. Some agricultural research investigates its derivatives for natural-product synthesis or pest deterrents. In my own work, setting up a route to these kinds of thiophenones proved useful for building molecular libraries that helped partners filter candidate drugs for further investigation.
The story of 2-oxo-3-methylthiophene in the lab hasn’t slowed. Many research groups look for efficient, green methods of assembling the core ring, cutting out harsh reagents and boosting yields. Academia finds interest in its derivatives as enzyme inhibitors and chemical sensors—areas where fine-tuned reactivity counts for a lot. Modern analytical techniques open up new views, letting researchers map out how this molecule assembles, reacts, or breaks down. Its presence in environmental samples sometimes guides pollution tracking near industrial sites. Technology transfer offices continue to field patent applications for both new synthesis routes and practical uses.
Work on the toxicity of 2-oxo-3-methylthiophene hasn’t been thorough enough to place it on major regulatory lists, but available data echo common themes from related compounds. Inhalation at higher concentrations causes discomfort in animal models, while ingestion shows mild acute toxicity in rodent studies. No comprehensive long-term studies exist for chronic effects, urging care among people handling it at scale. Most labs stick to using the lowest necessary amount and invest effort in sealed systems to minimize risk. Food chemistry research—particularly around flavor additives—focuses on tracking trace levels rather than bulk amounts, which shrinks possible consumer exposure to nearly negligible.
With the rise of green chemistry and targeted pharmaceuticals, 2-oxo-3-methylthiophene's role may keep evolving. Its structure supports further functionalization, matching the ongoing demand for reactive heterocycles in both industrial and medical chemistry. Advances in catalysis may one day allow nearly waste-free preparation, offering a more sustainable way to generate this and related molecules without old-school corrosive reagents. Ongoing developments in flavor science and electronics keep this structure relevant, and as screening technologies improve, so will the understanding of any toxicological challenges. This molecule proves how something small, with modest roots in academic chemistry, can steadily branch out across multiple disciplines and stay meaningful for decades.
Digging right in, the molecular formula for 2-Oxo-3-Methylthiophene is C5H4OS. A handful of carbon atoms, a dash of hydrogen, a splash of oxygen, and a sulfur atom make up this compact but interesting molecule. You probably won’t spot it on your grocery list or medicine cabinet, but chemistry folks know it as part of a family with a lot to say in laboratories and industry.
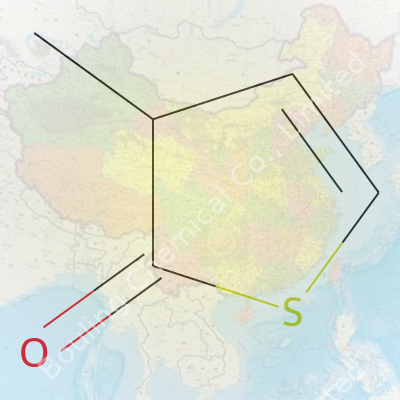
Imagine a five-sided ring. Three of those corners carry carbon atoms, one brings in oxygen (with a double bond), and the last houses sulfur. Stick a methyl group (which is just a carbon with three hydrogens) onto the third carbon in the ring, and you’ve got the skeleton of this molecule.
The structure looks like this: sulfur shares the ring with carbons, oxygen double bonds to the second carbon, and the methyl group hangs off the third. This setup gives it a distinct flavor, chemically speaking. In textbooks, drawings often show a pentagonal ring, with “S” and “O” written proud.
I remember the buzz in the lab the first time I came across this molecule. It came up during a project about flavor additives. Turns out, 2-Oxo-3-Methylthiophene—like several other substituted thiophenes—packs a punch for chemists tinkering with fragrances and flavors. Its sharp, nutty aroma slots it into work on roasted and meaty notes for food scientists trying to mimic grilled tastes in products.
Apart from its work in flavors, this molecule has a place in pharmaceuticals. With a stable ring and reactive spots, researchers explore its use as a building block when designing drugs or advanced materials. That said, you won’t find it in the vitamins aisle or your painkillers; its role is strictly behind the scenes so far.
Anyone who’s handled it knows lab safety matters here. Though its molecular formula looks simple, the presence of sulfur brings extra wrinkles. Many similar compounds sniff of rotten eggs or burnt matches, and some can irritate the skin or eyes. Proper gloves and ventilation — not just checkboxes, but daily habits in any half-decent chemistry workspace. Accessing small quantities from reputable suppliers avoids bigger headaches in purity and contamination.
Demand on a molecule like this remains steady, especially in food testing and drug experimentation. Scaling up safe synthesis helps stabilize costs. Smarter processes, maybe bio-based or less wasteful synthetic routes, could ease up the resource squeeze. There’s promise in using green chemistry to cut down toxic by-products, making smaller labs less wary of taking it on for new research.
Looking forward, companies betting on novel flavors or new materials could boost interest in derivatives like 2-Oxo-3-Methylthiophene. Teams who nail pure, scalable production without raising safety concerns will set the pace — not only in labs but also at the tasting bench and in future drug pipelines.
Most people never hear about 2-Oxo-3-Methylthiophene, yet this little chemical pops up in places you might not expect. In the world of pharmaceuticals, it shows value as a building block for bigger, complex molecules. Companies lean on it for drug discovery because its structure offers a shortcut to new treatments. The sulfur and oxygen ring can slip into other molecules without much fuss, giving drug designers extra tools to fight diseases like cancer or infections.
Back when I worked with chemists hunting for fresh antibiotics, I saw thiophene chemicals fill notebooks and lab benches. They wanted a clear path to efficient results, and small rings like this one let them build drug candidates quickly, often saving valuable time and resources when the pressure was on.
Walk through a fragrance lab, and you’ll notice shelves lined with small bottles labeled with tongue-twisting names. 2-Oxo-3-Methylthiophene sits on those shelves, waiting to add depth to scents and tastes. Chemists blend it into recipes for earthy or roasted notes—think coffee, cocoa, or grilled grain. That rich, slightly nutty smell some perfumes or foods offer comes from these kinds of molecules.
Food companies put trust in chemicals like this for natural flavor imitations. While not always used in large doses, a pinch can transform a product, helping baked snacks or drinks taste toasted or savory without actually roasting anything. In the early 2000s, I helped a snack company try out new seasoning blends. Adding a touch of these thiophenes upgraded the aroma, winning over focus groups that wanted “something warm, something real.”
Academic labs don’t skip over 2-Oxo-3-Methylthiophene, either. Graduate students and postdocs use it to make all sorts of custom chemicals that help them understand biological puzzles or improve electronic devices. Researchers say its ring-shaped structure interacts well during experiments studying the behavior of other compounds, so it works like a scaffold for creative problem-solving.
In the electronics field, the focus shifts to making more efficient organic materials. Scientists working on new semiconductors—think flexible smartphone screens or solar panels—reach for thiophene derivatives. They rely on the electronic properties that sulfur-containing rings bring, hoping for better conductivity and stability. The modern world leans harder every year on smarter, thinner electronics, so even slight performance jumps matter a lot.
Safe handling remains a big deal in any lab or factory using such chemicals. 2-Oxo-3-Methylthiophene demands good ventilation and careful storage. Its strong scent serves as a warning, but environmental safety teams ask for better solutions to keep waste out of water and air. Green chemistry could step up here—finding cleaner ways to make and recycle thiophene compounds, which will please regulators and neighbors living near manufacturing plants.
Price and supply chain stability challenge both big factories and small startups. If global trade hiccups or feedstock shortages hit, prices can spike. To guard against this, some companies now look for local suppliers or even try to produce key chemicals in-house. Their aim is to avoid nasty surprises and keep innovation on track.
2-Oxo-3-Methylthiophene, a mouthful for sure, serves as a building block for plenty of organic chemicals and fragrances. The sulfur brings in that distinctive sharp scent you’ll probably notice if you open a container in a small room. Its ease of reaction means you have to treat it carefully from day one, not just for safety, but to keep your batch in good shape for months down the line.
Planning to put away a few bottles of 2-Oxo-3-Methylthiophene? A dry, cool spot keeps it fresher and more stable. Most lab folks I know use tightly sealed amber glass. This shields it from light and moisture, which both cause chemical changes nobody wants. Ordinary room temperature often works, but if your climate runs hot or your lab’s HVAC isn’t perfect, a lower temperature (think: dedicated chemical fridge) only helps. Treat it like a bottle of good solvent or delicate essential oil — not something you want baking in the sun or sweating on a damp shelf.
The label stays critical. Re-label anything that loses its ink or adhesive. Mix-ups invite costly accidents or project delays. I’ve seen enough rushed storage lead to confusing situations and lost samples that everyone at my bench keeps a stack of fresh labels and markers handy.
Water sneaks in faster than most people imagine. If humidity wreaks havoc in your region, silica packs or desiccators make a difference. 2-Oxo-3-Methylthiophene likes to pull water and sometimes oxygen, especially if you leave it uncapped. Once moisture gets in, the sample can degrade or start growing mystery crystals. Good luck recovering product or keeping a reaction on track if you ignore basic dryness.
Cross-contamination with other reactive compounds brings trouble. Separate your oxidants, acids, and strong bases. I’ve learned this lesson watching a careless shelf-mate cause a bottle to puff up and vent—nothing dramatic, but enough to write off a week’s supply and shut down a project.
Gloves, goggles, and a fume hood sit at the front of every list. This chemical’s vapor can irritate eyes, skin, and lungs even at low amounts. Most folks who’ve splashed it or worked in a cramped space remember the sulfurous sting for hours. Using a pipette or dedicated spatula only for this bottle slows down cross-contamination or accidental spills. I’ve seen ruined balances and contaminated products from sharable scoops—separate tools save time cleaning up messes later.
Waste from runs with 2-Oxo-3-Methylthiophene goes in the special organic container, not down the drain. The sulfur content adds a burden to municipal treatments and might create hazardous vapors along the way. Read your facility’s disposal policies, since slipping up here puts everyone at risk and can trigger audits or worse. Keeping spill kits for small accidents lets you neutralize minor leaks promptly without calling for a full lab shutdown.
Starting with well-chosen containers, a dry and cool space, and real discipline about labels and separation pays off every time. Mixing sloppy routines with a compound this reactive ends up costing both safety and money. Simple good habits create a safer, more productive workbench, whether you’re making a small test batch or scaling up.
Anyone following the chemistry circuit or working in pharmaceuticals, pesticides, or dyes has come across 2-Oxo-3-Methylthiophene. This isn’t just another chemical with a complicated name and a certificate of analysis tucked away in some file. What matters here goes much deeper—boiling it down, this thing’s all about purity and specification. Most labs and suppliers hang their hats on making the grade, but not everyone takes the time to understand why a few invisible points on a purity scale really matter.
It’s easy to let your eyes glaze over when someone talks about 98% or 99% chemical purity, but I’ve seen rooms full of researchers sweat over that missing percent. For 2-Oxo-3-Methylthiophene, a standard lab-grade sample usually tops 98% purity, often written on the bottle as GC (gas chromatography) value. This single number can impact years of research. I remember one HPLC run thrown off just because a batch sat at 96.5%. The result: wasted reagent, wasted money, wasted time.
High purity means no surprises. Chromatography traces run smooth. End products get approval, not rejection. In pharmaceutical synthesis especially, hidden contaminants from lower purity batches can slip into final compounds. No one running clinical trials wants to explain a random peak on a chromatogram. Toxicity risks go up when specs get ignored, and that leads straight to regulatory headaches or, worse, product recalls.
Spec sheets aren’t just for compliance. For 2-Oxo-3-Methylthiophene, a typical datasheet reads: molecular formula C5H4OS, molar mass at 112.15 g/mol, melting point around 32–34°C, boiling point hovering around 185–187°C. Water solubility almost non-existent—hydrophobic as it gets. Color? Slightly yellow crystals or oil, depending on storage and humidity. Sometimes specs include a dryness limit since the compound can pull water from the air, turning a nice powder into a sticky mess that clogs up equipment.
Those aren’t just numbers. In practice, slight changes throw off final product qualities—color, stability, even shelf-life. That sticky mess I’ve seen gunk up a rotary evaporator or two. Producers try to dial specs in so their chemical fits the buyer’s needs, making it less about paperwork, more about real workflow efficiency.
Impurities in 2-Oxo-3-Methylthiophene aren’t just some academic point. Trace metals from manufacturing or unreacted starting material show up in analysis via NMR or GC-MS. These can start at fractions of a percent, but cumulative effects multiply downtime in any plant I’ve worked in. Residual solvents like dichloromethane sometimes stay behind if evaporation isn’t pushed hard enough. Those tend to ruin a whole batch in sensitive research or medical settings. Costs stack up. For QA people, every off-spec shipment means phone calls, back-and-forth, maybe a delayed product launch.
Reliable sourcing fixes half the problems. I’ve seen small firms save short-term cash with discount bulk suppliers, only to lose double in testing and reprocessing. Bigger names publish traceability paths for each lot, making specification part of their brand. Regular in-house sampling catches lags or contamination before it spreads downstream. Investing in checks instead of patching issues late always comes out cheaper.
At the end of the day, chemical purity and specification aren’t just numbers. Every industry relying on 2-Oxo-3-Methylthiophene needs sharp eyes and credible partners, because a molecule’s reputation rides on what you can’t see, as much as what you can.
Working in labs or chemical facilities, you come across many compounds with mouthful names like 2-Oxo-3-Methylthiophene. It’s not just another bottle sitting on the shelf. This compound carries risks that might surprise folks just getting into organic chemistry or industry work. You learn quickly that even a small spill or puff of vapor can lead to more than a bad smell in the lab. For me, the most useful safety lesson came after a colleague ended up with irritated skin just from handling chemical glassware that hadn’t been wiped down well enough. It drove home the point that one small misstep can blow up into hours spent in the safety shower or worse.
Nobody likes wearing goggles all day, but your eyes matter more than a little discomfort. Splash protection isn’t negotiable — not all splashes shoot up like Hollywood special effects, sometimes it’s a slow seep through gloves or a mist near your face. I watched a grad student learn this the hard way during extraction with volatile compounds, so I always reach for chemical splash goggles and not just standard safety glasses. Not all gloves hold up against organic solvents, so nitrile gloves, checked for tears and replaced after contact or a couple of hours, should be on every cart. Lab coats give another dependable barrier, and closed-toe shoes are more than dress code — one dropped flask turns them into lifesavers.
One detail that often gets skipped over in beginner labs involves fume hood use. I’ve worked in spaces where folks get lazy and just flip the sash up to dash something inside. Instead, keep that sash low and hands out — it shields you from the invisible vapors. 2-Oxo-3-Methylthiophene can let off fumes that bother the lungs and nose, even in rooms that smell only faintly of sulfur. Fume hoods also cut down on stray vapors lingering and contaminating other work surfaces. Don’t trust a room fan or a cracked window; these solutions don’t keep you out of harm’s way.
Clear labeling keeps people from making mistakes. I’ve seen chemicals stored under vague names or missing hazard labels, and sooner or later someone grabs the wrong container. Keep labels legible, include hazard symbols, and list the date opened so the age of the sample is obvious. Store 2-Oxo-3-Methylthiophene away from heat sources and incompatible chemicals like oxidizers to avoid surprise reactions. One time our team caught a storage mistake only because a sharp nose caught a strange smell, which could have ended badly.
Spills don’t wait for a convenient moment. I keep spill kits nearby and practice small-scale cleanup drills with coworkers. Absorbent pads, neutralizing agents, and waste bags need to be within reach, and everyone should know where the eyewash and safety shower stations stand. A clear plan helps reduce panic and mistakes. Medical help for exposure isn’t just for extreme cases: washing a small splash off right away beats waiting and hoping for the best.
Routine safety talks and refresher sessions turn into routines that stick. In group work, regular check-ins catch unsafe habits before they turn into accidents. Honest discussions about close calls build a safer work culture. Relying on common sense in the lab only covers so much — proper training arms everyone with smarter habits. It’s experience and vigilance paired with proper gear and good habits that help you keep working tomorrow, no matter what’s on today’s chemical list.
| Names | |
| Preferred IUPAC name | 3-Methylthiophene-2-one |
| Pronunciation | /tuː-ˈɒk.səʊ ˈθaɪ.əʊ.fin/ |
| Identifiers | |
| CAS Number | 638-38-0 |
| Beilstein Reference | 120928 |
| ChEBI | CHEBI:89277 |
| ChEMBL | CHEMBL16236 |
| ChemSpider | 162144 |
| DrugBank | DB03251 |
| ECHA InfoCard | 100.012.397 |
| EC Number | Not Assigned |
| Gmelin Reference | 792818 |
| KEGG | C19227 |
| MeSH | D016717 |
| PubChem CID | 13649 |
| RTECS number | KN6475000 |
| UNII | 0C6K8R1GDE |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | DTXSID2040173 |
| Properties | |
| Chemical formula | C5H4OS |
| Molar mass | 114.15 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | sulfurous |
| Density | 1.183 g/mL at 25 °C |
| Solubility in water | Slightly soluble |
| log P | 0.7 |
| Vapor pressure | 0.3 mmHg (25°C) |
| Acidity (pKa) | 7.20 |
| Basicity (pKb) | -5.4 |
| Magnetic susceptibility (χ) | -63.44·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.550 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.28 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 326.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 104.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2507 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H315 + H319 + H335 |
| Precautionary statements | Precautionary statements: P261, P264, P271, P273, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P337+P313, P362+P364, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | Flash point: 70°C |
| Autoignition temperature | 535°C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 320 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 2-Oxo-3-Methylthiophene: "800 mg/kg (rat, oral) |
| NIOSH | KK8225000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-Oxo-3-Methylthiophene: Not established |
| REL (Recommended) | 10 mg/m³ |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Thiophene 2-Methylthiophene 3-Methylthiophene 2-Oxothiophene 2-Acetylthiophene |