Looking back, chemists have always hunted for new building blocks that bend to their will—3-Bromo-2-Nitrothiophene popped up in the literature decades ago, soon finding praise in academic circles. The synthetic crowd first took an interest after realizing that simple tweaks to the thiophene ring could unlock a playground of fresh molecules. Researchers in the 1970s, placing value on both versatility and reactivity, put in the work, testing bromination and nitration routes to pick up this particular compound. Stories from senior chemists who actually handled the stuff describe a time when every new heterocycle held a promise of better drugs or switching circuits, so people really got their hands dirty refining preparations. The steady buzz around 3-Bromo-2-Nitrothiophene always circled back to its role as an accessible scaffold for further chemistry, long before automation and informatics turned the process digital. Each new derivative brought a sense of progress for those chasing better pharmaceuticals or functional materials.
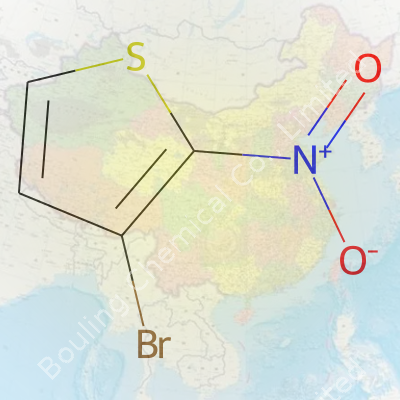
3-Bromo-2-Nitrothiophene features a five-membered thiophene ring holding tight to both a bromine atom and a nitro group. The presence of both groups on adjacent carbons means this molecule opens doors to modification, essential for folks working in synthetic organic labs. My own bench experience showed me how compounds like this act as stepping stones. Purity cuts down extra troubleshooting, so you barely have time to worry about tricky downstream reactions. Most suppliers today deliver it as a crystalline solid ranging in color from pale yellow to brown, packed in small bottles or ampoules with clear lot numbers and documentation. It arrives stable if kept out of bright sunlight and moisture. Since research budgets are always tight, bulk ordering sometimes tips the price, but purity and batch consistency mean fewer wasted hours on purification.
Solid at room temperature, 3-Bromo-2-Nitrothiophene melts shy of 80°C and sends up a distinct, sometimes pungent odor. Out in the open, high humidity can cause clumping, but stored tight, it keeps well for years. It dissolves in organic solvents with low polarity—think dichloromethane, chloroform, ether—not water, which helps with liquid-liquid extractions and quick purifications. The nitro and bromo groups both stand as flags to strong electrophiles, making the molecule reactive toward nucleophiles and certain metals. In practice, bench chemists call this mix of physical stability and chemical reactivity “user friendly,” so long as you treat it with the same respect as any nitro compound.
Labeling sticks close to international standards: you find molecular formula C4H2BrNO2, molar mass about 192.03 g/mol, batch date, and manufacturer’s address. Safety codes warn of toxic fumes under fire and the need for careful handling. Certified analyses list purity, usually above 97%, along with NMR and sometimes HPLC or GC traces. Labs that take documentation seriously include full material safety data sheets, hazard pictograms, and shelf life. This sort of transparency underpins trust between producer and scientist. I’ll say—from personal lab management headaches—clear specs shave days off troubleshooting and accidental exposure.
Preparation commonly starts with thiophene; bromination targets the second position using bromine in acetic acid at low temperature. Next, nitration introduces a nitro group at the third position, often via a mixed acid sequence. Over years, tweaks to solvent choice, reaction time, and separation steps have improved yields and cut down byproducts. Some research groups found ways to avoid byproduct isomers with precise temperature control and stepwise addition. Purer starting materials now mean fewer headaches later. Tedious purification often follows, using column chromatography to get rid of colored impurities. My own mentors drilled home the reality that patience during workup—slow filtration, thorough washing—pays dividends, leaving a tidy, highly pure product for downstream syntheses. Large-scale setups use jacketed reactors and in-line solvent washes, shrinking environmental footprint and worker exposure.
The bromo group acts as an open invitation for cross-coupling. Palladium-catalyzed Suzuki or Stille reactions let chemists snap on aryl or alkyl groups, with the nitro group holding firm as an electron-withdrawing anchor. For those aiming to build up molecular complexity, these modifications happen in a flask on any given Tuesday morning. The nitro group itself invites reductions—with iron and acid or catalytic hydrogenation—leading to amines that feed into dyes or pharmaceuticals. Careful substitutions near the sulfur atom build up an even broader set of possible compounds. In new functional material projects, this base molecule twists into advanced polymers or light-absorbing dyes. Colleagues who push the boundaries say the flexible backbone of this molecule gives creative minds room to work, provided they steer clear of overbromination or runaway reductions.
In catalogs and publications, 3-Bromo-2-Nitrothiophene also goes by 2-Nitro-3-Bromothiophene, 3-Bromo-2-Nitrothiofene, or simply as its CAS number, which happens to be 69387-98-6. Ingredient lists in regulatory submissions or specialty suppliers swap names freely, so experienced chemists train their eyes on both names and numbers. Once, I mixed up a reagent order because the vendor slipped in an “o-nitro” instead of “m-nitro”—one small typo, big headache. Synonyms keep the global supply chain in sync but demand careful double-checking.
No shortcuts in safety—nitro compounds like 3-Bromo-2-Nitrothiophene can irritate skin and lungs. Normal routine means full gloves, goggles, and lab coats; locals signs warn about dangers even in fume hoods with good airflow. Standard procedure isolates the compound from heat, open flame, and metal dust. In event of spill, use absorbent pads and seal the waste for prompt disposal. Experienced users store it below 25°C, out of the light, away from incompatible materials like strong reducing agents. Proper training on fire extinguishers and emergency eyewash stations protects lab coworkers. Mandatory documentation includes the latest GHS hazard codes, which supervisors check off before any bottle leaves the stockroom. The peace of mind from well-drilled safety drills and proper labeling can’t be overstated, especially late at night when only the sound of the refrigerator breaks the silence.
Pharmaceutical research relies on thiophene derivatives, using 3-Bromo-2-Nitrothiophene as both a starting block and building piece for more complex APIs. Agricultural chemistry exploits the molecule’s ease of further modification to fit new pesticides, herbicides, and fungicides. In materials science, scientists weave it into conductive polymers or specialty coatings for electronics. Lighting and sensor projects explore nitrothiophenes for their optical absorption, with some early-stage work touching on organic LEDs. As a grad student, I learned how a single molecule connects these distinct worlds: bench-scale patties of dry powder one day, promising preclinical hits or new device prototypes the next. The reach depends on clever minds and tough, methodical work—not just textbook reactions.
Active research focuses on new cross-coupling methods, aiming for greener conditions and higher selectivities. Some projects optimize yields, cutting down on toxic waste or boosting the number of possible functionalizations in a single pot. Reviews highlight how the electron-withdrawing nitro group unlocks selectivity in cycloadditions and radical reactions. Firms in pharma route small-scale parts of their pipelines through 3-Bromo-2-Nitrothiophene, customizing drugs for rare diseases or next-generation therapies. In my own experience reading patent databases, it turns up as a precursor for experimental CNS agents, antimicrobial scaffolds, and seeds for high-performance polymers. The open lanes for customization drive so much of the value in this space—one molecule, thousands of endpoints.
Reports point to moderate acute toxicity by inhalation or dermal exposure; nitro aromatics as a class raise concerns for hemolytic anemia or chronic organ effects with sustained handling. Animal studies remain sparse but caution persists—my industry trainers hammered home safe handling, trustworthy disposal, and regular health checks for anyone spending long hours with these materials. Nobody welcomes surprise symptoms after an innocuous spill. Ongoing studies probe for skin sensitization, environmental persistence, and safe thresholds under chronic exposure scenarios, pushing the field to adopt cleaner alternatives or reimagined workflows. Regulatory agencies keep a close watch, prodding for evidence and new best practices.
Prospects for 3-Bromo-2-Nitrothiophene ride on the hunger for new chemistries—smarter, cleaner, and adaptable. Academic labs pursue designer analogues to beat old inefficiencies; industrial teams scale up green methods to meet stricter rules. Discovery platforms now embed this molecule into digital design tools, aiming for lightning-quick optimization cycles. The hunt for functionalized thiophenes unlocks new patent claims, sharpened selectivities, and robust pharmacophores. Watching the markets, there’s little sign of slowing demand so long as innovation keeps pulling at the frontiers of medicine, material science, and sustainable manufacturing. My own take: the compound earns its shelf space, generation after generation, by rewarding researchers with both reliable chemistry and fresh promise for what science can build.
Chemists often dig into the specifics of molecules, and for good reason. Each atom adds character. Take 3-Bromo-2-Nitrothiophene. Roll that name around, and you’re looking at a molecule that brings together bromine, a nitro group, and thiophene’s sulfur-rich ring. Its molecular formula looks like this: C4H2BrNO2S. That tells the story—four carbons, two hydrogens, a bromine atom, one nitrogen, two oxygens, and a single sulfur.
For chemists, knowing the code—these formulas—is like having a molecular recipe. The recipe opens doors to new reactions, new materials, even new ways fight disease or tackle pollution. We live in a world made of molecules. Making sense of them starts here, with formulas and numbers.
Knowing what goes into a molecule lets you work out how much it weighs. We call it the molar mass or molecular weight. With 3-Bromo-2-Nitrothiophene, you tally up each atom’s contribution:
Total those up, and you land on a molecular weight of about 176.05 g/mol. Precision counts, especially for lab work. Weighing out the right amount means fewer wasted chemicals and less frustration. Maybe it even means discovering a new way to use a molecule like this.
Many folks outside of labs glance at molecular numbers and tune out. Inside the lab, that string of letters and numbers means predictable behavior. With 3-Bromo-2-Nitrothiophene, you’re likely dealing with something reactive. Bromine atoms love to swap spots when nudged. Nitro groups shape a molecule’s electric personality, making it useful in a wave of reactions that spark everything from pharmaceuticals to dyes.
I’ve watched chemists light up over the possibilities tucked inside these numbers. Job-wise, knowing weights and formulas stops accidents and confusion before they start. That tiny gap between 1.7 grams and 17 grams of a chemical isn’t a typo—it’s a difference you notice the moment you pour a powder into a solvent, and everything either fizzes, sits still, or erupts in a mess.
Everyone likes to believe their spreadsheet or calculator always delivers the answer. Double-checking math and chemical data should be a habit, not an afterthought. Sifting through supplier catalogs, formulas shorten the hunt. Instead of guessing at sample identity or purity, you match the formula. Mix-ups drop, work speeds up.
There’s a trick in memorizing these small details—structure, weight, formula—if you work with organics daily. I keep a notepad or digital table handy. Seeing those numbers often enough, they turn familiar. They become shortcuts to confidence, not just within the four walls of a lab, but across the many hands and voices that trade these chemicals around the world.
If the science classroom made you roll your eyes at molecular weight tables, step back and see what those numbers enable. They save time. They boost safety. They fuel new inventions. In my experience, giving formulas and weights the respect they deserve pays off, whether you’re a research veteran or a chemistry student just cutting your teeth on thiophene and its friends.
Anyone working in a chemistry lab for more than a week has a favorite shelf of quirky aromatic compounds. 3-Bromo-2-nitrothiophene has earned its own spot. With both a bromine and a nitro group loaded onto a sulfur-containing ring, this chemical looks pretty specialized at first glance. It’s not something you just pour into a beaker and wait for magic to happen. Yet, researchers keep coming back to it, and there are solid reasons why.
Most people outside synthetic chemistry don’t give much thought to how their medicines start out. Many common drugs trace their way back to heterocyclic rings—the kind where sulfur, nitrogen, or oxygen sticks into the backbone. Researchers use 3-bromo-2-nitrothiophene to create those rings, giving them plenty of sites to add or swap pieces. The bromine atom is especially handy for Suzuki or Sonogashira couplings, forming carbon-carbon bonds with precision. That sort of trick allows chemists to link up other rings or bulky side chains, a step that pops up again and again in medicinal chemistry.
The nitro group offers its own set of options. Chemists often reduce it to an amino group, which acts as another anchor for adding new fragments. Looking back through dozens of journal articles, it’s clear this compound helps produce antibiotics, anti-inflammatory drugs, and even some compounds that show promise against cancer cells. In my graduate years, we once managed to knock out three different scaffolds for new drug candidates out of a single batch of this precursor. The efficiency surprised everyone in the lab.
Interest in organic electronics keeps rising each year. Materials with sulfur-containing rings show electrical characteristics missing in many old-school, petroleum-based polymers. 3-Bromo-2-nitrothiophene pops up in the synthesis of organic semiconductors and conductive polymers. Its structure invites fine-tuning, where both electronic and physical properties can be controlled by swapping out groups or connecting the thiophene ring to larger molecular systems.
Take the design of next-generation light-emitting diodes and solar cells. Researchers can start with 3-bromo-2-nitrothiophene when building complex backbone structures. The electronics industry bets on such high-end compounds because regular silicon has its limits; flexible screens and lightweight solar panels demand clever chemical engineering. Experiments in our university’s materials lab showed that small tweaks in precursors like this lead to big shifts in conductivity and light absorption—evidence for how vital this building block can be.
Agriculture doesn’t often grab flashy chemistry headlines, yet a ton of modern crop protection starts in small-scale organic synthesis. 3-Bromo-2-nitrothiophene stands out as a precursor for certain fungicides and insecticides. The electron-withdrawing nitro group influences a compound’s biological activity, helping tailor molecules that are tough enough to last in the field but break down in safe ways after doing the job.
Through collaborations with agrochemical startups, I saw more junior chemists than I could count work through dozens of analogs. Many experiments failed, but a few hits carried through to the trial stage each season. Tweaking the base building blocks, including 3-bromo-2-nitrothiophene, sharpened results and provided alternatives when resistance started to show up.
No honest review of synthetic building blocks should ignore their environmental footprint. Production methods still rely on harsh reagents, and safer, cleaner synthesis deserves priority. Promising steps involve exploring catalytic processes that cut waste and energy use. Open discussions with chemical suppliers help push industry standards in the right direction. My own move toward greener chemistry started with skepticism, but pilot projects using newer methods succeeded, and that kind of progress puts useful compounds like this within reach for future generations without the same old hazards.
Anyone working in a chemistry lab knows the routine: you set up your experiment, everything seems under control, and just as you’re about to wrap up, someone asks if that small bottle of 3-Bromo-2-Nitrothiophene got stored correctly. That question isn’t a formality. The storage of this organic compound matters to personal safety, product stability, and the success of future experiments. Sometimes a shortcut leads to ruined chemicals or, worse, serious health hazards. I’ve seen near-misses in real labs because someone stacked incompatible reagents or left volatile organics alongside open flames, and that taught me most problems start with careless storage.
3-Bromo-2-Nitrothiophene isn’t your typical household product. It brings its own set of risks because of the bromine and nitro groups. These groups mean two key things: don’t let it near heat and don’t let moisture mess with it. I store bottles like this in a cool, dry cabinet, away from direct sunlight or temperature swings. Easy to say, but I’ve worked in labs where the “cool” room sat near an old-school radiator, and just a few degrees made all the difference. Everyday heat sources, like nearby exhaust vents or computers, raise the temperature. That can lead to slow decomposition, and sometimes, that creates those nasty byproducts nobody wants to clean up.
Humidity deserves attention too. Even if the compound looks stable in the bottle, moisture invites clumping, hydrolysis, or even worse reactions if contaminated with acids or bases. So a proper desiccator, or at least silica gel packs, is critical. At my last job, a wet cabinet from a leaky pipe destroyed half a shelf of delicate reagents because the team hadn’t checked recently. Checking seals on containers sounds boring, but that’s what kept our sensitive chemicals safe through every season.
A tight-fitting cap is more than a convenience. Fumes from bromine-based organics spread fast, and leaks turn a quiet lab into a safety drill. I always double-check that each bottle seals tight, nothing sticky on the threads, nothing cracked around the cap. I’ve learned to respect warning labels—if the bottle looks worn or the label starts fading, it’s time to print a new one. Mislabeling or fading ink leads to mistakes, and I once had to help a colleague figure out what a mystery powder was after years of bad habits. Now, regular inventory checks keep the whole shelf in order.
Mixing reactive chemicals is a recipe for surprise fire drills. I separate halogenated, nitro, and oxidizing compounds from each other, giving risky items their own space. Putting 3-Bromo-2-Nitrothiophene next to acids, strong reducing agents, or oxidizers is asking for trouble. Small, clear storage bins or trays inside the cabinet serve as a safety net so spills stay contained. In one busy teaching lab, I watched as careless stacking led to a spill that could’ve ruined more than just the experiment—good organization made all the difference when we cleaned up.
The best storage routine for 3-Bromo-2-Nitrothiophene boils down to a simple approach: keep it cool, keep it dry, seal it tight, label it well, and don’t stack it with danger. Mistakes in storage may not produce drama every day, but I’ve seen enough real-life lessons to know that vigilance beats complacency every single time. Doing the right thing protects people, experiments, and pocketbooks. If you want your reagents ready for action, don’t cut corners on storage.
Most folks working in synthetic chemistry meet all sorts of finicky, volatile compounds. Anyone who’s had a bottle of 3-bromo-2-nitrothiophene in their hands probably recalls the nervous glances at its pale yellow crystals and that unique, not-so-kind aroma. There’s almost always a question hanging overhead: is this stuff going to act up just sitting on the bench, or can I trust it between uses?
A bromine and a nitro group share space on a thiophene ring. Chemistry tells us that stacking up electron-withdrawing groups like nitro and bromo can crank up reactivity or, in some cases, create structural tension. That’s what gets chemists worrying about bottles popping or powders turning brown without a clear reason. The truth is, stability doesn’t just come out of a textbook; it comes from quite a bit of collective sweating and caution tape in the lab.
I've pulled this compound from the back of storage shelves more than once. The material holds up well in sealed glass, away from sunlight and moisture, at room temperature. No fuming, no sudden explosions, no sticky mess. On the other hand, tossing it into the open air or letting the cap sit loose brings risk. Nitro groups enjoy reacting with strong acids, bases, and reducing agents. Things turn sour fast if it gets wet or if someone thinks it’ll survive a hot flask. It’s not as delicate as diazomethane or as notorious as picric acid, but it sure ranks above typical lab solvents.
There’s a very real history of accidents tied to nothing more dramatic than bad luck and poor planning. I’ve seen glass vials crack because storing near a radiator bumped the temp above 40°C. Dried-out 3-bromo-2-nitrothiophene survives in the short run but will degrade if humidity creeps in. Breakdown products aren’t always as benign as we’d like. It’s easy to brush off “store in a cool, dry place” as mere formality until a stinky surprise breaks up a workflow.
Across the industry, 3-bromo-2-nitrothiophene doesn’t show up on major explosion or runaway lists. Yet, its structure deserves respect. Chemical supply catalogs highlight its dangers, listing it as harmful, irritant, and environmentally toxic. I take cues from seasoned researchers: small batches, tight seals, desiccators when possible. Routine checks for caking, color change, or leaks catch problems early. One bad batch can derail a project or rack up serious fines for lab violations.
Nobody wants to sound like a broken record, repeating lab safety protocols. But 3-bromo-2-nitrothiophene isn’t just another shelf filler. Good labels, up-to-date Safety Data Sheets, and rigorous housekeeping spare everyone trouble later on. Simple tweaks, like swapping open-top jars for screw-caps and adding silica gel packets to storage bins, protect hundreds of dollars’ worth of chemicals and keep labs incident-free. It might not be the wildest compound in the storeroom, but regular vigilance keeps experiments on track and everyone out of trouble.
3-Bromo-2-Nitrothiophene sounds like an exotic molecule, but it’s the kind of thing researchers regularly work with in organic synthesis labs. It packs quite a punch: high reactivity and a structure that gives off warning bells for both health and environmental risks.
Nitro groups and halogenated thiophenes each bring their own dangers. Nitro compounds in general release toxic fumes if they decompose or burn, and halogenated organics won’t do your lungs any favors, either. Those who handle chemicals every day know what happens when someone gets too casual: unexpected reactions and accidents lead to everything from skin burns to respiratory troubles. The compound’s reactivity means it can irritate skin, eyes, and lungs, so direct contact or breathing in dust or vapors is something to avoid.
Spraying a splash of strong-smelling nitro compounds in a fume hood quickly teaches you to respect lab safety. Once, I noticed a slight yellow dust film near a balance after weighing a similar compound—just a hint told me gloves and gloves alone weren’t enough; masks, splash goggles, and careful cleanup matter. Accidental contact led to irritation, and careless storage led to a surprise shelf leak days later. There’s a reason so many researchers develop ‘lab hands’—dry, red skin comes easy where aggressive reagents and lax attention cross paths.
Using 3-Bromo-2-Nitrothiophene begins with checking ventilation. Fume hoods keep fumes and dust away from your face. For personal protection, I wear nitrile gloves, long sleeves, splash-proof goggles, and, on sniff-test days, a proper chemical respirator—cloth masks won’t cut it. Lab coats give an extra layer that catches any splashes before they hit skin or clothes.
Spills bring on the most risk. Loose powder on the benchtop or in a weighing station is easy to sweep into the air, so damp paper towels for cleanup and immediate disposal work better than dry dusters. Proper waste containers labeled for halogenated organics keep disposal clear—no tossing things straight into the trash.
Storing this stuff in tightly sealed, well-labeled containers limits how much air and moisture get in and stops accidental mix-ups. Cool, dry storage away from acids, bases, and reducing agents avoids unwanted reactions. If a container cracks or leaks, many labs have spill kits with neutralizing agents, absorbent pads, and instructions that go beyond a simple water rinse.
No one should feel afraid to ask for help or clarification on safety. A quick review of the Safety Data Sheet (SDS) brings important reminders for first aid—like flushing eyes for at least 15 minutes if exposed, or using emergency showers for skin contact. I’d rather look foolish by double-checking than end up with a serious injury.
A lot comes down to habit. If you keep your work area tidy and never rush through a synthesis or cleanup, risk goes down. Regular training and honesty—admitting when you don’t understand a risk—also keep dangers in check. Outsiders may roll their eyes at all the steps, but everyone in the lab values being able to go home healthy at the end of the day.
| Names | |
| Preferred IUPAC name | 3-bromo-2-nitrothiophene |
| Other names |
2-Nitro-3-bromothiophene 3-Bromo-2-nitro-thiophene 3-Bromo-2-nitrothiophene |
| Pronunciation | /ˈθaɪ.əˌfiːn/ |
| Identifiers | |
| CAS Number | 39856-61-6 |
| 3D model (JSmol) | `3D Model (JSmol) string` for **3-Bromo-2-Nitrothiophene**: ``` C1=CSC(=C1Br)[N+](=O)[O-] ``` |
| Beilstein Reference | 1368736 |
| ChEBI | CHEBI:91138 |
| ChEMBL | CHEMBL3306379 |
| ChemSpider | 160332 |
| DrugBank | DB04264 |
| ECHA InfoCard | 100.023.935 |
| EC Number | 33819-77-1 |
| Gmelin Reference | 104243 |
| KEGG | C19109 |
| MeSH | D017860 |
| PubChem CID | 124407 |
| RTECS number | WS6460000 |
| UNII | J0A0J0NW3L |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C4H2BrNO2S |
| Molar mass | 221.06 g/mol |
| Appearance | Pale yellow to light brown solid |
| Density | 1.96 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.97 |
| Vapor pressure | 0.00361 mmHg at 25°C |
| Acidity (pKa) | 1.17 |
| Basicity (pKb) | 9.6 |
| Magnetic susceptibility (χ) | -49.2e-6 cm³/mol |
| Refractive index (nD) | 1.624 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.68 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 341.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -5.70 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1082.2 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Danger |
| Hazard statements | H301, H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P304+P340, P312 |
| Flash point | 70°C |
| Lethal dose or concentration | LD50 (oral, rat): >2000 mg/kg |
| NIOSH | SN3675000 |
| PEL (Permissible) | N/L |
| REL (Recommended) | 0.1 ppm |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
2-Nitrothiophene 3-Bromothiophene 2-Bromothiophene 3-Nitrothiophene 2,3-Dibromothiophene 2-Bromo-3-nitrothiophene |