Chemists started noticing the potential of benzothiazole derivatives back in the early twentieth century. The search for newer rubber vulcanization accelerators and additives drove this push, because older chemicals had safety and efficiency problems. As the field moved into the 1950s and 1960s, morpholine groups caught the eye of researchers, who added them to existing frameworks and produced compounds like 2-(Morpholinothio)Benzothiazole. Practical studies soon followed, tracking the effect of these tweaks on industrial applications. Each time an additive like this landed in factories and labs, conversations flared — people wanted clean reactions, reliable performance, and fewer headaches from toxicity or instability. This instinct doesn’t fade. Each batch of a new accelerator or additive pulls from this heritage, testing old ideas in new contexts.
2-(Morpholinothio)Benzothiazole stands out among similar accelerators because it pulls double duty as both an accelerator and a stabilizer in rubber processing. Chemical companies slot this compound into catalogs under categories like rubber accelerators or vulcanization agents. In the market, you see it referenced alongside MBT or MOR, and sometimes as MBS or MORBT, all depending on the supplier and country. For practical workers in rubber mixing plants, 2-(Morpholinothio)Benzothiazole offers predictability and speed, dealing capably with raw rubber and fillers to produce smoother products.
The powder or crystalline form carries a slightly earthy chemical smell, off-white or light yellow. The melting point hovers near 88–94°C, so it doesn’t risk decomposition at typical processing temperatures but doesn’t need extreme heat to mix in. This makes the compound easier to handle during blending and production. It doesn’t dissolve in water but shows reasonable solubility in organic solvents like benzene or acetone, which means workers can dissolve or mix it based on what serves their batch chemistry best. Molecular weight lands around 264.37 g/mol, and its structure (a morpholine ring attached via sulfur to a benzothiazole) creates some stability that other accelerators don’t always show.
Product labels depend on jurisdiction, but good practice sees chemical manufacturers listing purity (often 96% or higher), batch number, storage suggestions, and clear warnings about personal protective equipment. Technical data sheets specify physical state, melting point, pH stability, bulk density (generally about 1.3 g/cm³), and handling. In shipping and storage, UN numbers and hazard codes matter for regulatory compliance. Labels highlight acute toxicity and skin/eye irritation potential, drawing a line between industrial-scale handling and ordinary consumer use.
Production usually starts with 2-mercaptobenzothiazole and morpholine. The manufacturer combines them, often under mild heat and solvent, with the reaction typically forming the thioether linkage. After completion, the producer cools the mixture and extracts the product—often washing and recrystallizing it to remove byproducts. From trial and observation, this method stays relatively straightforward, using equipment common in basic chemical manufacturing. Choice of solvents and purification steps can make or break batch consistency, and this is one of those places where technical experience pays off big, since a little impurity causes downstream headaches.
2-(Morpholinothio)Benzothiazole reacts with reducers or strong acids under certain conditions, especially if the sulfur bridge can be disrupted. Further, the morpholine group opens routes for quaternization or N-alkylation, which researchers sometimes exploit when customizing the accelerator properties for specific rubber types or crosslinking requirements. These reactions allow for the design of tailored derivatives, depending on whether higher activity or slower curing times serve the product at hand.
Across chemical suppliers and literature, you see a parade of aliases: MOR, MBS, MORBT, 2-(Morpholinothio)benzothiazole, or even CAS 95-31-8. Some catalogs stick with the IUPAC name, while trade paperwork or shipping manifests use simple abbreviations. Users still need to double-check the CAS number and synonyms to ensure substitutions don’t lead to the wrong ingredient in an industrial batch.
Personal safety is no afterthought here. Toxicity shows up in animal studies and regulatory documents, which warn of risk through skin contact, inhalation, or accidental ingestion. On factory floors or in research labs, we require respirators and gloves. Spills demand prompt cleanup, especially since fine powders may disperse. MSDS sheets underline fire-fighting measures because, in a blaze, decomposition creates nasty surprises—sulfur or nitrogen oxides among them. Any waste stream carrying this chemical falls under hazardous material laws, and regular worker training bridges the gap between compliance on paper and safety on the job.
Rubber curing and compounding define where this compound contributes most. It accelerates the cross-linking of sulfur with unsaturated rubber, influencing how tire treads, gaskets, belts, or even footwear turn out. Some research looks for applications in specialty elastomers or high-performance industrial goods, because tweaks to cure speed and crosslink density make a big impact in demanding environments. Some advanced formulations look outside tires. Medical devices, cable insulation, and shock-absorbing materials all benefit from the reliable accelerant properties, even if supply chain staff still track each truckload’s compliance certificate.
Academic and industrial labs rarely run out of questions. With rubber blends evolving—alloys with nano-fillers, sustainable feedstocks, or heat-sensitive plastics—chemists circle back to accelerators like 2-(Morpholinothio)Benzothiazole. Researchers keep testing how new processing approaches, like lower-temperature curing or microwave vulcanization, react to traditional and novel chemical additives. Some work pushes for hybrid accelerators or multi-function agents, designed to lower toxicity while keeping processing speed. These research projects don’t stay confined to academic publications. They swing quickly back to pilot plants and full-scale factories.
Decades of toxicity studies show the risks are real. Acute toxicity to rodents happens at moderate doses, prompting classification under REACH and GHS chemical safety frameworks. Skin contact sometimes produces dermatitis; animal studies clock reproductive and developmental toxicity at certain dose levels. These findings have led regulators in Europe and Asia to demand stronger warning labels and storage practices. Even so, occupational exposure levels don’t typically top out above recommended thresholds in facilities with basic engineering controls and procedural training, but the emphasis on improvement never lets up.
The drive for safer, greener accelerators shapes the future for 2-(Morpholinothio)Benzothiazole. Bio-based elastomers are gaining market share, yet manufacturers still rely on established accelerators for high-value, technically challenging applications. The pressure now comes from customers and regulators chasing reduced toxicity and eco-friendlier end-of-life disposal. Researchers look to new functional group modifications, computational predictive models, and lower-toxicity analogs. Some labs try encapsulation technologies, hoping to minimize occupational exposure and environmental persistence without losing the accelerator’s technical advantage. Whether or not a clear-cut “green” substitute emerges in the next decade, the history and proven record of benzothiazole derivatives—alongside continued investment in process safety and formulation science—will keep them in the conversation for years.
Speak with anyone who’s worked in a tire plant, and you’ll hear about the constant push for safer and longer-lasting parts. 2-(Morpholinothio)Benzothiazole has turned into a regular ingredient behind the scenes. The compound gets mixed into rubber recipes for a simple reason: it helps sulfur create cross-links between the rubber molecules during the heat-curing process known as vulcanization. This cross-linking isn’t just technical lingo. It really determines how the tire holds up after miles of pounding the pavement—whether a kid’s bike tire or the wheels under heavy-duty trucks.
When I walked the floor of a local tire manufacturer outside Cleveland, engineers told me just how tricky achieving the right balance can be. They want a tire that stays strong when hitting potholes. They also want to avoid cracks from forming after years of use. The organosulfur structure in this chemical makes it dependably reactive with sulfur, so the end product flexes yet resists breaking down from heat and weather.
Big factories run their conveyor belts across shifts and seasons. The staff can’t afford a belt that snaps, so companies look for compounds that support durability. The compound shows up here too, in the rubber coating those belts, or in the seals that hold back oil and grease. If these rubber pieces fail, there’s usually downtime—something every plant manager hates. Adding 2-(Morpholinothio)Benzothiazole builds in a buffer against wear and tough industrial environments.
Companies face pressure from global supply chains to keep equipment running smoothly. The cost of routine breakdowns adds up. This chemical offers a safeguard, so belts and seals run closer to their expected lifespan. For example, in the world of food processing or mining, equipment may run around-the-clock, and the risk of hot oil or high-speed friction makes the right vulcanization agent a necessity.
Tire makers are not the only group leaning on 2-(Morpholinothio)Benzothiazole. Chemists working on EPDM (ethylene propylene diene monomer) and other synthetic rubber types use it to fine-tune properties for specific products. Think weather stripping around car doors, window seals, or anti-vibration pads under heavy machines.
Regulations in the automotive and construction world set strict benchmarks for weather resistance and longevity. If you look through technical papers, you’ll spot references to the compound helping materials pass these tests. Its ability to work at lower curing temperatures reduces production costs and makes the process safer, limiting the fumes that could harm workers. This practical side of manufacturing doesn’t always get headlines, but it shapes the way products perform in the field.
Safety and environmental impact have become big topics in chemical production. Researchers continue pushing for alternatives with fewer health risks, but there’s no quick replacement for the decades-long track record behind 2-(Morpholinothio)Benzothiazole. More companies now monitor worker exposure and fine particles in the air. Better ventilation in plants and strict labeling keep things above board.
Finding balance between toughness and environmental responsibility is the next step. Investing in cleaner production and supporting worker education builds trust in this critical part of the modern rubber industry.
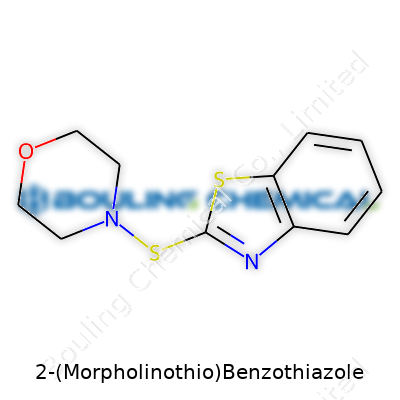
Chemistry loves big names, and 2-(Morpholinothio)benzothiazole proves the point. Strip away the jargon, and you find a molecule that catches attention in both laboratory circles and factories. Its backbone carries a benzothiazole ring—a familiar sight in the world of organic chemistry. Imagine two joined rings, one with nitrogen and sulfur tucked inside. At position two of this ring system, a special group dangles: the morpholinothio bunch.
Morpholine kicks in with its own ring, shaped by four carbons, a nitrogen, and an oxygen. The “thio” tells us sulfur creates the bridge linking morpholine to the benzothiazole. You end up with a landscape that’s part science puzzle, part industrial tool.
Add up the atoms, and you see why 2-(Morpholinothio)benzothiazole interests chemists. The molecular formula lays out as C11H12N2OS2. Eleven carbons, twelve hydrogens, two nitrogens, one oxygen, and two sulfurs. These pieces come together in a very specific arrangement, not just a random scatter. This arrangement shapes how the molecule behaves — reacts in the lab, stands up under manufacturing heat, mixes in with other substances.
The chemical structure gives it multiple personalities. At one end, the benzothiazole brings stability and a knack for handling heat. The morpholinothio side wants to bond with other chemicals, unlocking new possibilities for making products stronger, more flexible, or longer-lasting. That’s why you find this compound written into the recipe for rubber accelerators and additives.
The rubber industry leans heavily on compounds like this. In tires, belts, hoses, and a lot more, rubber has to perform under stress, sunlight, and temperature swings. 2-(Morpholinothio)benzothiazole boosts vulcanization, the step that transforms sticky, raw rubber into a durable, bouncy material. This molecule speeds up the process and produces a tough, resilient rubber. Time after time, we count on it for safe driving, sturdy soles, dependable hoses, and seals that don’t give up early.
Rubber parts keep cars running and factories moving. Using effective accelerators lowers energy needs and cuts waste. This ties straight into sustainability. Manufacturing gets cleaner, and products last longer.
Handling chemicals always brings health and safety into the discussion. 2-(Morpholinothio)benzothiazole sits on lists that require attention to workplace guidelines. Gloves, ventilation, and protective eyewear aren’t optional—they keep workers healthy. Tracking and reducing waste protects waterways and communities.
Scientists keep looking for ways to use less of the hazardous stuff, or swap in greener alternatives without sacrificing performance. Conversations have started between regulators, manufacturers, and researchers to drive improvements. It all points in the same direction—cleaner production, safer job sites, less impact after use.
Chemicals like 2-(Morpholinothio)benzothiazole shape things you handle every day. The science behind them matters, as does the way companies handle risks and environmental responsibilities. Learning the gritty details of a molecule’s structure, understanding what those atoms do, isn’t dry textbook learning—it’s the key to safer industries and better products. With new research and open collaboration, there’s a clear path toward smarter choices that pay off on roads, in workplaces, and for public health.
Safe storage of chemicals isn’t just a technical step—it shapes routine safety and can affect the quality of any subsequent application. There’s a good reason workers don’t stash sensitive chemicals on an open shelf or ignore temperature swings. Years of lab work have taught me that even a small slip in storage practices sometimes results in ruined material, wasted money, or worse, a health incident.
2-(Morpholinothio)Benzothiazole isn’t something anyone should treat with casual care. It’s used widely in rubber manufacturing, and like many benzothiazole derivatives, it calls for a healthy respect. If you ever spilled a volatile compound on your bench, you know how quickly something minor can escalate. Even minor contamination from humidity, heat, or incompatible surroundings can change the properties of a compound or trigger dangerous reactions.
This compound wants a dry, cool home. Moisture in the air isn’t its friend—water can speed up hydrolysis and result in chemical breakdown. I’ve seen a container that sat unsealed in a humid storeroom turn lumpy and off-color in a matter of weeks. Dry conditions matter more than plenty of folks realize, and silica gel packets or desiccators offer a simple fix.
Temperature swings pose another risk. Most suppliers advise keeping the product at temperatures below 25°C (77°F). Storing chemical inventory near a furnace vent or on a sunlit window ledge is an accident waiting to happen. Heat can speed up unwanted reactions—and even though this compound isn’t the most volatile, every degree over the recommended range shaves time off its usable life.
Another routine I always follow: tightly sealed containers. Oxygen and dust don’t belong in the container. The reaction of benzothiazole derivatives with oxidizers isn’t just theoretical—it happens. Leaving the lid off, even for a short time, invites contaminants that can degrade the chemical or pose harm. Using airtight, clearly labeled bottles or drums and closing them fast makes a difference.
Storing everything together might seem like a space saver, but it’s a shortcut that’s landed people in trouble before. 2-(Morpholinothio)Benzothiazole doesn’t do well sitting next to oxidizing agents, strong acids, or bases. A mislabeled jar or a splash from an incompatible fluid can spark reactions. Keeping dangerous substances apart isn’t just regulation—it’s practical wisdom passed down from folks who’ve seen it go wrong.
Many facilities use color-coded storage racks or clear dividers in cabinets. That helps workers identify and separate risk groups quickly, which makes life easier during inspections or emergencies. Labels showing hazard classes, storage temperatures, and incompatibilities stick in your mind and help prevent mistakes.
Working in the field, you learn not to trust memory alone—gloves, goggles, and lab coats become second nature. I’ve noticed that most accidents happen when people skip steps because the process feels routine. Regular training on storage procedures, spill protocols, and emergency action plans forms the backbone of chemical safety in every lab or warehouse.
Modern tracking systems can help—barcodes and digital logs offer real-time inventory checks so expired or mishandled agents get flagged early. Simple changes—a backup thermometer in the storage area or routine checks for moisture—go a long way. This approach doesn’t just follow rules; it reflects a culture of common-sense care and responsibility that experts and newcomers can both appreciate.
2-(Morpholinothio)Benzothiazole, widely used in the rubber industry as an accelerator, shows up in work environments more often than most folks realize. It’s a mouthful of a name but in practice, it’s a fine powder or crystalline solid that demands respect. I’ve seen enough cases where casual attitudes turn dangerous, particularly in small workshops or local labs, where safety culture sometimes slips in favor of speed or convenience.
Many hazards come from underestimating prolonged exposure. Dust from this compound can irritate the eyes and skin, sometimes causing allergic reactions in sensitive people. Once, after a rushed unloading job, one team member developed a nasty rash just by brushing powder off his sleeves. Inhaling dust or fumes—whether you’re mixing large batches or just cleaning up—hits the respiratory tract fast. For some, the coughing doesn’t start immediately, which can make the risk easy to brush off until it’s too late. Even worse, regular exposure may lead to chronic breathing problems, especially in ill-ventilated workspaces.
Beyond bodily harm, there’s the concern of what happens to leftover material and waste. Washing powder down the drain, tossing rags in the regular trash—these habits may seem minor, but over time they add up. Local waterways show traces of industrial chemicals like this one, putting fish and plant life at risk. When we handle hazardous compounds without thought for runoff and proper disposal, it’s not just our shop or factory that suffers; the impact ripples outward.
Dealing with this chemical safely always starts with personal protective equipment. Standard gear means gloves, safety goggles, long sleeves, and a respirator. I keep extra sets of gloves around since worn or torn gloves lose their value in seconds. A single careless touch to the forehead or face makes all the precautions pointless.
Proper ventilation cannot be stressed enough. Open windows won’t cut it. Mechanical exhaust fans and local fume extraction systems make a real difference. In one facility, the installation of fume hoods cut down on incidents by half in just a few months.
Lifting and transferring this powder, even weighing small amounts, stirs up more dust than one expects. Wet methods—using damp towels to clean up minor spills rather than dry sweeping—keep the powder out of the air and out of people’s lungs. Training staff, not just handing them the rulebook, helps reinforce these practices. I’ve seen seasoned workers pick up new tricks in demonstration sessions, realizing that shortcuts aren’t worth the risk.
Responsible chemical handling includes labeling every container—too many times, I’ve seen people trust memory, only to forget which container holds which substance weeks later. Keeping track with regular inventory checks and lockable chemical storage keeps things organized and cuts down on accidental exposure. Waste disposal deserves just as much attention. Arranging for specialized chemical disposal and maintaining a log of what gets thrown out, and when, creates accountability and protects both people and the local environment.
Simple respect for the hazards involved, paired with the right equipment and clear routines, means workers make it home safely, businesses avoid legal trouble, and the environment stays cleaner. Safe handling isn’t just about checking boxes; it’s about understanding the real costs when safety doesn’t come first.
Anyone who's spent real time in a lab—or any industrial setting—knows the headaches that pop up when purity or packaging don’t line up with your research or production goals. 2-(Morpholinothio)Benzothiazole might sound like a mouthful, but for tire manufacturers and some specialty rubber goods, it’s a staple. I’ve seen more teams frustrated by procurement problems than by technical questions. The real-world details, like how pure your chemical needs to be and what size barrel or bag you actually need, shape results, budgets, and even lab safety.
Purity levels drive quality and performance. Some labs swear by 98% and up, especially in R&D. Others get what they need well below that, because their process tolerates traces of impurities. Picking the wrong grade hits you in the wallet, or worse, throws off results. I remember a colleague working on a polymer blend who switched sources unknowingly. Batch after batch, the final product flaked. Tracing it back, the culprit turned out to be a new source with a lower, inconsistent grade. Only after digging into certificates of analysis did the issue get sorted.
In industrial circles, cost pressures sometimes push buyers to accept slightly less pure material, trading a few dollars per kilo for possible process headaches down the road. Lab teams fight this, but management needs proof! This isn’t just nitpicking. According to published technical notes from several specialty chemical suppliers, purity fluctuations as minor as 1-2% send certain rubber formulas off spec, causing measurable differences in aging and flexibility.
Packaging choices sound mundane, yet they can make or break a workflow. A chemist in a high-volume operation orders in drums. A university lab needs just a few grams, often for one-off experiments. Piling up excess because you had no choice but to buy 25 kilos doesn’t make sense for most. Besides the cost, extra stock means higher accident risk, harder inventory tracking, and real headaches for waste disposal. In my experience, good suppliers offer a range—from one-liter bottles or bags up to 200-liter drums. Some even provide custom splits.
Shipping rules for chemicals like 2-(Morpholinothio)Benzothiazole add another wrinkle. Smaller packs sometimes simplify legal compliance and cut down on storage paperwork, especially for labs squeezed by regulations. Nobody wants to waste half their grant money or time navigating hazmat red tape.
Trustworthy suppliers back up their claims with documentation. This means batch-level Certificates of Analysis, safety data sheets, and frank answers about any differences in purity or packaging. I watch for firms that don’t dance around tough questions. In one frustrating procurement cycle, I spent weeks just trying to nail down exactly what I’d be receiving. I’ve learned to keep a running list of suppliers who send everything up front, saving me time and resources.
The bottom line for anyone needing 2-(Morpholinothio)Benzothiazole: push your supplier for exactly what you need. Ask about tailored quantities. Double-check purity before you sign anything. Ten minutes up front, asking the right questions, saves months of headaches later. Technical specs are only half the battle; user experience, price transparency, and solid documentation carry the rest. In my work, those small advantages have meant fewer ruined batches, less waste, and happy bosses—not to mention a safer lab bench.
| Names | |
| Preferred IUPAC name | 2-(1,4-oxazin-4-ylsulfanyl)-1,3-benzothiazole |
| Other names |
MBTMO 2-(Morpholin-4-ylthio)benzothiazole |
| Pronunciation | /tuː ˌmɔːrˈfoʊlɪn.oʊˈθaɪ.oʊ ˌbɛnzoʊˈθaɪ.əˌzoʊl/ |
| Identifiers | |
| CAS Number | 95-07-8 |
| 3D model (JSmol) | ``` 3D;JSmol|30|ball-and-stick|1|C1=CC=C2C(=C1)N=C(S2)SN3CCOCC3 ``` |
| Beilstein Reference | 1647988 |
| ChEBI | CHEBI:85464 |
| ChEMBL | CHEMBL486022 |
| ChemSpider | 120689 |
| DrugBank | DB08414 |
| ECHA InfoCard | 03a6a28a-5c30-4fa3-aaa4-86a827667e97 |
| EC Number | 239-487-1 |
| Gmelin Reference | Gmelin 84157 |
| KEGG | C19072 |
| MeSH | D08.811.277.352.650.225.100 |
| PubChem CID | 85513 |
| RTECS number | GL9100000 |
| UNII | UI3G60C37D |
| UN number | 2811 |
| CompTox Dashboard (EPA) | DTXSID5020187 |
| Properties | |
| Chemical formula | C11H12N2OS2 |
| Molar mass | 267.38 g/mol |
| Appearance | Light yellow liquid |
| Odor | Odorless |
| Density | 1.37 g/cm3 |
| Solubility in water | Insoluble |
| log P | 1.93 |
| Vapor pressure | 0.000013 hPa (25 °C) |
| Acidity (pKa) | 4.73 |
| Basicity (pKb) | 6.89 |
| Magnetic susceptibility (χ) | -72.49e-6 cm³/mol |
| Refractive index (nD) | 1.661 |
| Viscosity | Viscous liquid |
| Dipole moment | 4.56 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 208.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -962 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. Toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261-P273-P280-P305+P351+P338-P337+P313 |
| NFPA 704 (fire diamond) | 2-(Morpholinothio)Benzothiazole: NFPA 704: 2-1-0 |
| Flash point | > 193.5°C |
| Autoignition temperature | Autoignition temperature: 380°C |
| Lethal dose or concentration | LD50 oral rat 600 mg/kg |
| LD50 (median dose) | LD50 (median dose): 727 mg/kg (rat, oral) |
| NIOSH | RN822-11-1 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 mg/m³ |
| Related compounds | |
| Related compounds |
2-(Morpholinoamino)benzothiazole 2-(Morpholinylmethyl)benzothiazole 2-Chlorobenzothiazole 2-Mercaptobenzothiazole 2-(Piperidinothio)benzothiazole |