Chemistry owes much to the steady drive of researchers-led curiosity, and 2-Morpholinoethylamine came out of that sort of determined work. Decades ago, manufacturers and scientists were searching for compounds that could help build smarter drugs and advanced materials. The morpholine ring, discovered long before, gave rise to derivatives like 2-Morpholinoethylamine. Through the last half of the twentieth century, improvements in synthetic techniques and analytical tools allowed chemists to isolate and study these small amines in greater detail. The compound quickly found its place in pharmaceutical labs and industrial research as a handy intermediate, helping chemists test new reactions and probing the boundaries of molecular biology.
People working with specialty chemicals run into 2-Morpholinoethylamine under several trade names and product codes in global catalogs. The compound, recognized by its distinct molecular structure—a morpholine ring tethered by a two-carbon linker to an amine—offers a combination of reactivity and stability. Chemical suppliers stock the compound in liquid form, usually in amber bottles that keep out light, helping maintain its shelf life. Companies provide different grades, with varying purity, since lab protocols can require anything from technical to ultra-pure varieties. Its niche status means only a handful of major chemical distributors handle it in bulk, but smaller research shops and university stores still demand small batches for their custom syntheses.
Looking at a bottle of 2-Morpholinoethylamine, you handle a clear, viscous liquid that carries an amine-like fishy odor—a reminder that even small organic molecules have personalities all their own. Its boiling point sits above 200°C, which speaks to the stability afforded by the morpholine ring. With a melting point usually below room temperature, the compound remains manageable under normal conditions. The amine group provides a route for protonation, while the morpholine moiety boosts hydrophilicity, making the compound soluble in common solvents, especially water and alcohols. Chemists like its ability to act as both a nucleophile and a base, making it a flexible tool in organic reactions.
Labs often require documentation for compounds to check identity and purity. Suppliers label bottles with the molecular formula, CAS number, lot or batch codes, recommended storage temperature, and hazard symbols. Material safety data sheets (MSDS) describe the potential health effects and disposal methods, and buyers receive a certificate of analysis with every significant order. Purity—measured by gas chromatography or NMR spectroscopy—becomes a talking point, with pharmaceutical labs insisting on 99%+ grades and more forgiving applications accepting technical-grade material. Such standards help labs maintain reproducibility, especially in regulated industries where a single bad batch could affect months of work.
Making 2-Morpholinoethylamine usually starts with a reaction between morpholine and a two-carbon alkyl halide like 2-chloroethylamine hydrochloride. This nucleophilic substitution, run in a basic aqueous environment, often uses phase-transfer conditions or organic solvents to keep yields high and byproducts low. Industrial setups might add purification steps, using distillation or liquid-liquid extraction, to ensure product quality. Even in a small lab, careful control of temperature and reaction time goes a long way toward boosting product yield and minimizing side-products. This pathway, first mapped out decades ago, persists thanks to its simplicity and accessibility of raw materials.
Chemists value 2-Morpholinoethylamine for its versatility in chemical transformations. The primary amino group allows acylation, alkylation, and reductive amination, making the molecule useful in synthesizing pharmaceuticals and custom ligands. The morpholine ring tolerates a range of reaction conditions, resisting oxidation and hydrolysis under moderate settings. Functionalization of either the amine or the ring itself leads to derivatives that expand its use in drug design. It reacts cleanly with carboxylic acids, isocyanates, and sulfonyl chlorides, and can serve as a linker or bridging group in the assembly of bioconjugates. This adaptability means research chemists keep a bottle handy for projects that go off the beaten track.
Ask around in different labs and you’ll hear 2-Morpholinoethylamine called by several other names. Some refer to it as N-(2-Aminoethyl)morpholine or morpholinoethylamine, depending on their background. Chemical suppliers often list synonyms on labels and safety data sheets to make cross-referencing easier, especially since international regulations vary in naming conventions. Catalog listings highlight common identifiers, such as the CAS number 931-27-3, so that researchers can order with confidence and avoid costly mistakes. Some proprietary blends and specialty formulations feature the compound under brand names, but in the literature, the chemical name usually dominates.
Like many amines, 2-Morpholinoethylamine requires careful handling. Direct skin contact can cause irritation, while inhalation of vapors may lead to headaches or respiratory tract discomfort. Facilities operating with the compound install fume hoods and provide gloves and goggles, and chemical hygiene plans outline proper procedures for spills and disposal. The compound is not listed among the most dangerous industrial amines, but respecting its reactivity and potential for harm keeps workers safe. Local regulations about storage, labeling, and transportation also influence how companies handle the compound from delivery to waste management.
Research labs and manufacturers see 2-Morpholinoethylamine as valuable in several fields. Medicinal chemists use it in the assembly of potential drug candidates, where the morpholine group signals improved pharmacokinetics or increased water solubility. The compound serves as a building block in agrochemical synthesis, sometimes surfacing in formulations that enhance crop protection. In analytical chemistry, it functions as a derivatizing agent, helping highlight specific functional groups during detection. Its solubility and reactivity expand its use into specialty polymers and coatings, where custom performance profiles matter more than mass-market appeal. Industrial applications remain relatively narrow, but creative chemists continue to push its boundaries.
Academic and industrial research teams explore new chemistry using 2-Morpholinoethylamine, focusing on better reaction pathways and safer applications. One major trend looks at green chemistry: reducing solvent waste, using milder reaction conditions, and swapping out hazardous reagents. Another trend taps into biological testing, where the compound’s derivatives show promise as enzyme inhibitors, neurotransmitter analogs, or targeted molecular probes. Many research groups publish on improved synthetic routes from renewable feedstocks, reducing the industry’s dependence on fossil fuels. Co-developments with universities spark new patents and experimental therapies, showing just how versatile this building block can be when clever minds get to work.
Every chemical compound faces scrutiny over its health effects, and 2-Morpholinoethylamine is no exception. Toxicological studies in animals and cell cultures reveal modest acute toxicity, with higher concentrations leading to organ stress and changes in blood chemistry. Chronic exposure studies highlight the importance of controlling workplace environment, as repeated inhalation or ingestion could trigger longer-term effects on liver and kidney function. Safety sheets recommend minimizing exposure and avoiding release into the environment, since water solubility raises concerns about potential groundwater contamination. Research continues, especially since regulatory guidelines update as new evidence emerges and occupational health agencies learn more about low-dose, long-term risks.
Science always looks ahead, and 2-Morpholinoethylamine stands ready for broader use and new forms of molecular innovation. The future offers opportunities in pharmaceutical research, where tailored analogs could unlock smarter therapies for neurological disorders or cancer. As polymer technology searches for novel reactants, this amine’s structure brings new properties for advanced materials that must balance flexibility with strength. Ongoing work in green chemistry might soon deliver manufacturing routes that cut waste and energy use, reducing the compound’s environmental impact. Regulatory standards keep tightening, meaning companies will need reliable toxicity data to maintain compliance and protect public health. As chemical synthesis enters an era of tailored molecules, compounds like 2-Morpholinoethylamine shape what tomorrow’s science and industry can achieve.
2-Morpholinoethylamine might not show up in everyday conversations, but anyone who has spent time around a chemistry lab understands chemicals like this drive a lot of quiet innovations. Scientists, researchers, and folks working with specialty processes rely on it for more than just textbook chemistry.
From my years spent shoulder-to-shoulder with lab personnel, I’ve seen this molecule show up in surprising places. It’s not part of a recipe you keep under your kitchen sink or a name you find on common ingredient lists. In research labs, this clear, colorless liquid acts like a building block. It helps create more complex molecules used for many different projects—think pharmaceutical research, paint chemistry, or specialized plastics.
The power of 2-Morpholinoethylamine comes from the little things it can do inside a reaction flask. Its structure gives it both basic and nucleophilic qualities, which, in plain terms, means it can snug up to other molecules and start chain reactions chemists are after. It plays a big role in helping create compounds used for medicinal chemistry—early trials for new drugs, for example. Many successful medications didn’t start off as eureka moments, but from days and nights spent hunting for the best pieces to fit together. This chemical ended up in a few of those puzzle boxes.
Anyone in the materials field knows innovation pushes demand for unique, high-performing compounds. Building a polymer that resists scratches or chemicals requires small-molecule intermediates that hold everything together. 2-Morpholinoethylamine gets mixed in, not for its name or reputation, but for how it helps achieve tough engineering goals. I’ve watched friends in coating development count on it while testing water-resistant blends or adhesives that don’t budge.
Some researchers use 2-Morpholinoethylamine to anchor other molecules, making surfaces that stick only when allowed—think biomedical devices or smart materials. The cost of switching away from time-tested building blocks can run high. Labs that test alternatives often land right back at familiar molecules like this one, since the proof—strong results and safe testing data—already exists.
Not every chemical belonging to a lab shelf should head into public view. 2-Morpholinoethylamine calls for careful handling. Just because it mixes well with water and solvents doesn’t mean it’s safe for skin or airways. Lab teams follow strict rules when pouring or mixing, wearing gloves, goggles, and keeping fume hoods working. From my hands-on lab stints, small mistakes bring quick reminders: a cough, an itchy wrist, and a missed lunch as you rinse irritation away.
The bigger discussion touches on responsibility. Anyone working with specialty chemicals owes it to colleagues to keep safety data sheets close and review protocols. This isn’t just red tape; it keeps accidents down and research up. Reviewing toxicity and disposal guidelines often shows how prepared you are, not how experienced you claim to be.
If industries run into trouble sourcing 2-Morpholinoethylamine—or struggle to replace it—they usually hit dead ends faster than expected. Some suppliers keep stocks flowing, but sudden shortages raise costs and stall projects. Open communication between manufacturers, safety regulators, and researchers helps avoid nasty surprises.
More universities and public research groups have started building resource-sharing programs. Posting best practices or sharing alternative synthesis routes gets real value out in the open. This builds trust, not just among chemists, but with anyone who depends on the next generation of medicines, new plastics, or coatings.
2-Morpholinoethylamine often shows up in labs where research pushes the boundaries of pharmaceuticals and advanced materials. Its structure stands out for its blend of organic chemistry basics and functional groups that unlock multiple uses in real-world applications.
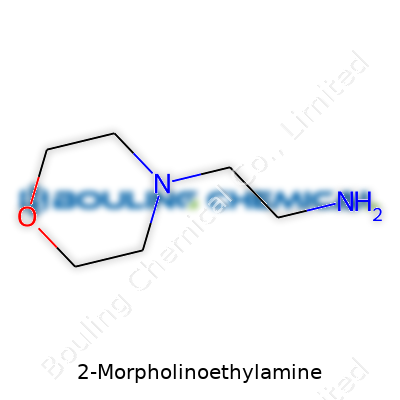
At the core, 2-Morpholinoethylamine contains two main features. There’s a morpholine ring—a six-membered ring made of four carbon atoms, one oxygen atom, and one nitrogen atom. This ring grabs the attention of chemists because its specific arrangement leads to stability and the ability to react in planned ways. Connected to this ring, you’ll find an ethyl chain (two carbons long) that ends with an amine group (–NH2).
The molecular formula looks like C6H14N2O. To picture 2-Morpholinoethylamine, imagine the morpholine ring acting like a rounded house, with the nitrogen in the ring linking through a two-carbon chain to that –NH2 group. That setup gives it a hand in both water-based and oil-based chemistry, which makes it valuable for drug discovery and custom chemical synthesis.
Every piece of this molecule plays a role. The morpholine ring behaves much like a flexible base, allowing the compound to slip into reactions where both mild acidity and mild basicity come into play. This adaptability goes a long way for researchers developing new medicines—especially in programs looking to improve how drugs dissolve and get absorbed. The terminal amine group offers reactivity, making it easy for chemists to attach other chemical pieces or to create bonds with other molecules.
From personal work in the chemical industry, I’ve seen how this kind of chemical framework gets used for designing lab tools and probes. The morpholine ring resists breakdown and offers just the right size to fit into biological systems. That opens up the option to use 2-Morpholinoethylamine when working on drugs that target enzymes or when building molecules meant to pass smoothly through cell layers.
According to PubChem—a trusted database maintained by the National Institutes of Health—2-Morpholinoethylamine sits at a place in research where both pharmaceutical chemistry and polymer development find it useful (PubChem CID: 12337). It often pops up as a starting material for synthesizing more advanced molecules, including those that help treat diseases or control how polymers behave.
The World Health Organization’s guidelines emphasize safety for chemicals with secondary amine groups like this one because they might react in unexpected ways under certain conditions. By following these guidelines, labs keep their work safe and produce more trustworthy results.
Concerns show up most often around safe storage and handling. 2-Morpholinoethylamine draws up a sharp odor, and the amine group makes it best to use protective gear and proper ventilation. Labs can cut down on risks by setting up clearly marked work areas and collecting real-time air data, so workers know if vapors reach unsafe levels.
Teaching younger chemists about the reasons behind each safety step gets results. From my own experience, walking through a synthesis while demonstrating safe transfer, neutralization, and waste disposal creates a habit of responsibility that sticks—a lesson worth repeating as new chemicals with similar structures hit the scene.
Working with 2-Morpholinoethylamine isn’t much different from dealing with other amines, but you won’t go far if you treat it like any household chemical. The minute an amine aroma leaks, noses start burning. This chemical comes as a clear, colorless liquid. It can irritate the skin and eyes. Inhaling its vapors can also tickle your airways and make breathing difficult. Getting too comfortable in a lab packed with volatile amines has never done anyone any favors.
Chemical-resistant gloves, lab coats, and safety goggles have saved a lot of folks from regretting sloppy habits. Direct skin contact with this amine leaves a sting and can quickly lead to dermatitis or worse. I remember a colleague who brushed some off a benchtop with his bare arm — he was scratching for days. The fix is simple: wear gloves made of nitrile or neoprene, and strip them off before touching doorknobs or your face.
Working in a well-ventilated fume hood goes a long way. Even a brief exposure to the vapor can make you cough, so open bench transfers aren’t the way to go. Good ventilation clears out vapors and keeps headaches off your daily to-do list. Face shields help if there’s any chance of splashing. A pair of safety glasses won’t cut it if you’re mixing up larger batches.
2-Morpholinoethylamine reacts with acids and oxidizers. That bottle shouldn’t share shelf space with corrosive acids or bleach. Anybody who’s worked in a tight prep room knows messy storage leaves you with more cleanup, and sometimes surprises. Use tightly sealed containers and label them clearly. Always store the chemical away from direct sunlight or heating vents. This keeps pressure from building in the bottle and avoids leaks.
Small spills in my early days taught me that amines travel fast through gloves and across worktops. Absorb any spill with vermiculite or a similar inert material, scoop it up with care, and toss it in a sealed chemical waste bag. Never try to mop up chemical spills with your regular cleaning rags. Any rags soaked with amines end up stinking and can become a fire risk.
Washing down the drain sends amine odor through the plumbing in old labs, causing complaints from everybody, including maintenance. All liquid waste should go to chemical disposal, following local rules. Most labs have specific bins for amine waste because mixing in incompatible chemicals can make for dangerous reactions, even in trash.
Quick, thorough action beats panic. If 2-Morpholinoethylamine gets on your skin, wash it off with soap and a lot of water for at least 15 minutes. If splashed in eyes, rinse at the eyewash fountain and call a colleague for help. Breathing in high concentrations? Get straight to fresh air, and don’t try to shake it off — call for medical help.
Chemistry doesn’t reward lone rangers. Peer checks, up-to-date safety sheets, and proper training make it much less likely that someone will walk away with a burn or headache. Following the handling and storage tips above has kept my lab running clean and safe, and kept the air clear — for both new students and the old-timers alike.
ReferencesLooking at 2-Morpholinoethylamine, the sheet shows its molecular formula: C6H14N2O. Getting the molecular weight feels pretty straightforward, but it’s worth giving a quick explanation. Add up the atomic weights for each element. Carbon: 12.01, hydrogen: 1.01, nitrogen: 14.01, and oxygen: 16.00. Six carbons, 14 hydrogens, two nitrogens, and one oxygen—doing the math lands at 130.19 g/mol. For folks who work with chemicals, grabbing the right number makes all the difference, whether you’re mixing a reaction by hand in a small lab or scaling up to something with pumps and huge tanks.
Anyone who has spent hours at a lab bench knows the impact of a single decimal point. The molecular weight of 2-Morpholinoethylamine determines how much to weigh out for solution prep, stock concentrations, and even transport documents. Even pharmaceutical and biotechnology companies rely on these numbers. Slipping up at this step doesn’t just mess with a batch—it can upend a whole series of experiments and even cost a company a regulatory headache.
2-Morpholinoethylamine isn’t a household name, but it shows up in a fair few places. Its structure offers up both an amine and a morpholine ring, giving it edge in polymer chemistry, biochemistry, and drug discovery. Chemists prize its flexibility to link other molecules or serve as a starting block for new compounds. The molecule gets plugged into research chasing new treatments and sometimes, new materials with specific traits. Here, a solid handle on molecular weight lets researchers plan reactions, scale their work, and meet product specs without tripping into costly waste or do-overs.
Plenty of newcomers fall into the trap of misreading chemical names—there’s no shame in double-checking research papers or material data sheets. With chemicals like 2-Morpholinoethylamine, a quick check with a trusted source like PubChem or Sigma-Aldrich can spare someone from running incorrect experiments or preparing solutions that just won’t work. Some labs write the molecular weight on their bottles with a marker to sidestep confusion. Experience teaches that mistakes can stack up quickly, and tracking details from the start always pays off.
Accurate documentation saves more than just time. Whether in academic research or industry production, it pays to have procedures that make double-checking standard. Reliable digital scales get calibrated weekly, and staff receive regular training on chemical handling and safety gigs. Consistency matters, both for scientific progress and for safety. For those jumping into the field, leaning on digital tools and databases can fill gaps in knowledge and stop errors before they cost time or, worse, safety incidents.
Modern labs run lean, and the tech in use keeps stepping up—but precision never goes out of style. The molecular weight of 2-Morpholinoethylamine clocks in at 130.19 g/mol, but every new task starts with that one fact. Keeping things accurate from the first step passes benefits through the whole project, no matter how small it looks at the start. Working with real numbers, not guesses, turns chemistry from a gamble into a craft.
Walking into any chemistry lab, you can feel the quiet tension between productivity and safety. Something as unassuming as 2-Morpholinoethylamine can change everything if handled carelessly. It’s a clear liquid, deceptively simple, yet a whiff in the wrong place or an accidental spill brings out its potent, corrosive nature. For those who’ve worked with amines, stories about headaches, skin burns, and fire hazards aren’t new. Just about anyone handling this compound learns early: storage isn’t just a checklist, it’s survival.
Let’s talk containers first. I never trust thin-walled plastic. Glass is tempting, but some grades crack, especially with temperature swings or rough handling. Tested, high-density polyethylene offers a solid middle ground. A tight-fit cap, no leaky seals, is crucial. A forgotten drip on the bench can mean a ruined afternoon and a mandatory safety report.
Direct sunlight raises risk for two reasons. Heat speeds up decomposition, which can release harmful vapors, and UV light can cause yellowing or even unplanned reactions. A dark, temperature-stable cabinet with enough ventilation handles both heat and fume build-up. I once saw a storage room double as a closet—row of chemicals next to someone’s lunch. That’s an invitation to disaster. Storing 2-Morpholinoethylamine apart from food, paper, or anyone’s belongings isn’t bureaucracy, it’s how you keep accidents away.
Mixing this amine with strong acids creates heat and potentially hazardous gases. Strong oxidizers are a bigger risk; they can ignite an unnoticed drop. No one needs that kind of emergency on a regular Thursday afternoon. Putting this compound in a dedicated space, away from acids, oxidizers, and incompatible solvents, cuts through a lot of the risk. Remembering those simple labels—acid cabinet, organic section, base locker—saves more than paperwork. It keeps lives and careers intact.
I’ve seen well-meaning folks wrap bottles in plastic bags “just in case.” Moisture seeps in, causing corrosion or pressure build-up. Every chemistry instructor will argue for honest, legible labeling. Scratched or faded labels don’t protect anybody. I make it a habit to update storage logs as soon as a new batch arrives. Spot checks mean nobody is caught off guard.
Ventilation seems boring—until a spill sends vapors right into your face. Local exhaust or at least a fume hood cuts down exposure. For high-volume use or crowded labs, a separate, ventilated chemical storage unit helps everyone breathe easier. Safety showers, eyewash stations, and gloves sit near storage for a reason. Cotton won’t help much; nitrile gloves and thick goggles stand up to this chemical’s bite.
Everyone wants to leave the lab in better shape than they found it. For 2-Morpholinoethylamine, storage means thinking ahead. Regular checks, clear policies about what goes where, and refusing shortcuts allow science to move forward without unnecessary risk. Online safety databases provide up-to-date guidelines, and reaching out to manufacturer reps or safety consultants isn’t just a formality—it’s solid risk management.
| Names | |
| Preferred IUPAC name | 2-(Morpholin-4-yl)ethan-1-amine |
| Other names |
2-Aminoethyl morpholine N-(2-Aminoethyl)morpholine Morpholine, 2-aminoethyl- |
| Pronunciation | /tuː-mɔːrˌfiːl.oʊˈɛθ.əlˌæm.iːn/ |
| Identifiers | |
| CAS Number | 6237-06-3 |
| Beilstein Reference | 1637992 |
| ChEBI | CHEBI:17218 |
| ChEMBL | CHEMBL372123 |
| ChemSpider | 34723 |
| DrugBank | DB08308 |
| ECHA InfoCard | 100.057.844 |
| EC Number | EC 203-899-9 |
| Gmelin Reference | 85288 |
| KEGG | C06025 |
| MeSH | D019307 |
| PubChem CID | 18147 |
| RTECS number | MP1400000 |
| UNII | IV927MQK5G |
| UN number | UN2735 |
| CompTox Dashboard (EPA) | Q27144086 |
| Properties | |
| Chemical formula | C6H16N2O |
| Molar mass | 130.19 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Ammonia-like |
| Density | 0.997 g/mL |
| Solubility in water | Miscible |
| log P | -1.08 |
| Vapor pressure | 0.17 mmHg (25°C) |
| Acidity (pKa) | 9.85 |
| Basicity (pKb) | 5.80 |
| Refractive index (nD) | 1.484 |
| Viscosity | 6 cP (20°C) |
| Dipole moment | 2.48 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 322.96 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -104.6 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06,GHS05 |
| Signal word | Warning |
| Hazard statements | H302, H314 |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 62 °C |
| Autoignition temperature | 215 °C (419 °F; 488 K) |
| Explosive limits | Lower: 1.4% Upper: 12.4% |
| Lethal dose or concentration | LD50 (oral, rat): 1,790 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 1730 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg |
| Related compounds | |
| Related compounds |
Morpholine Ethanolamine N-Methylethanolamine Diethanolamine Morpholinoacetic acid |