Decades back, labs focused their attention on amino alcohols for better solubility and versatile chemical behavior. Among this group, 2-Morpholinoethanol soon earned a spot in research circles for its balanced properties. As industries adopted more water-based systems and greener chemistry, this compound moved up the ranks, driven by demand for a chemical that could fit hybrid roles—both as a building block and performance additive. Early patents show 2-Morpholinoethanol as a common feature in corrosion inhibitor blends and specialty surfactant systems. Technology shifts encouraged manufacturers to refine synthesis, reduce impurities and respond to regulatory updates, shaping its current profile and application reach.
Inside most chemical supplier catalogs, you can find 2-Morpholinoethanol listed for its amine-alcohol hybrid structure. Chemists prize it as more than a raw material—the compound steps into roles from emulsifier to neutralizing agent, even working in paint stabilization and as a reagent in organic synthesis labs. For formulators chasing persistent solubility or pH control, this chemical delivers performance at moderate concentrations. Decades of field and lab trials show it runs efficiently without strong odor or volatility issues, and with proper handling, storage, and basic training, it enters production lines smoothly.
Under standard lab lights, 2-Morpholinoethanol appears as a colorless to pale yellow liquid, flowing with medium viscosity. Its mild amine scent blends into formulations, rarely overwhelming more volatile elements. The chemical structure—blending a morpholine ring and ethanol sidechain—produces solubility in water and organic solvents, making it a go-to for hybrid aqueous-organic systems. Melting point sits well below freezing, while boiling point clears 200°C, lending thermal stability for coatings or resins requiring heat resistance during cure. In standard SDS sheets you’ll see flash points around 116°C, and the chemical blends with acids or oxides, often acting as a mild base, without the harsh impact of stronger amines.
Quality 2-Morpholinoethanol arrives with tight purity specs—usually above 99% for industrial grades. Impurities such as water, other morpholine derivatives, and residual solvents draw scrutiny during batch release. Labels emphasize CAS number 622-40-2, UN shipping codes, and required GHS hazard symbols. Container drum sizes differ from smaller lab bottles to heavy-duty 200-liter barrels, often constructed from HDPE or steel with appropriate seals to prevent leakage or contamination. Certificates of Analysis travel with every batch, outlining density, refractive index, color (Hazen scale), and GC purity so users can check conformity before use in high-value or regulated processes.
Chemical engineers usually prepare 2-Morpholinoethanol by reacting morpholine with ethylene oxide in a controlled reactor, monitoring temperature and pressure to favor the mono-alkylated product. The route steers clear of heavy metal catalysts, limiting risk for downstream impurities. Aqueous work-up and distillation steps follow to yield a clean product. Larger producers might recycle unreacted morpholine, squeezing out better atom economy and lessening waste stream load. Each process tweak focuses on cost, yield, and minimizing worker exposure to volatile intermediates. Modern plants automate feed additions and detect exotherms, keeping process safety in tight check.
With both secondary amine and alcohol groups in its makeup, 2-Morpholinoethanol adapts in synthetic schemes. The alcohol end goes through esterification, etherification, or opens doors for polymer cross-linking. Chemists often draw on the amine function for quaternization, introducing hydrophilicity or anchoring the molecule into larger surfactant frameworks. Reacting with isocyanates gives rise to urethane derivatives, while acyl chlorides prompt amide formation. Some research circles experiment with metal complexation for catalyst design, taking advantage of coordinated nitrogen and oxygen. The molecule’s bifunctional nature means a single drum can suit both formulation and synthetic experimentation—a cost saver in many operations.
In catalogs and MSDS forms, you might run across alternate names like N-(2-Hydroxyethyl)morpholine, 2-(Morpholin-4-yl)ethanol, or simply Morpholine ethanol. The diversity in product naming reflects historical sourcing and differing conventions across regions. Trade names can carry the prefix "Morpho" or "Aminoethanol", yet all trace back to that morpholine-ethanol core. In regulatory filings or transport docs, CAS number 622-40-2 ensures clarity for logistics, safety, and compliance.
Handling 2-Morpholinoethanol takes respect for its chemical nature; skin and eye protection top the PPE list. Spills prompt rapid response—ventilated areas and spill kits designed for amine-containing liquids. GHS labeling points out irritant risks, and ingestion cases pull in poison control experts due to mild toxicity. Storage stays away from acids, oxidizers, or open flames, leveraging chemical stability but not taking unnecessary risks near reactive partners. OSHA, REACH, and similar authorities all review data, shaping practices in transport and on-site use; actual plant and warehouse setups vary but good housekeeping keeps liberated fumes and accidental mixing at bay. Regular respirator checks and eyewash stations become standard in higher-throughput facilities.
2-Morpholinoethanol’s mainstay roles show up across paints and coatings, where its amine function stabilizes resins and balances pH. In metalworking, it forms a backbone in corrosion inhibitors, defending steel surfaces from acidic wet environments. Textile auxiliaries pick up its mild amine bite for dye solubilization and fabric softening. In water treatment, its blend of hydrophilicity and mild basicity neutralizes acids, reducing fouling and optimizing ion exchange performance. Research labs frequently use it to introduce morpholine character into novel drug candidates, bioconjugates, or catalysts. Semiconductors and electronics plants trust the chemical for specialty cleaning baths, thanks to solvency and low residue.
From university benches to corporate pilot plants, 2-Morpholinoethanol underpins new surfactant design, ion-exchange resins, and advanced coatings. Recent years brought exploration of bio-derived morpholine routes, promising smaller carbon footprints. Pharmaceutically inclined labs regard it as a versatile motif, pushing SAR studies where morpholine rings unlock new activity in enzyme inhibitors or CNS-active agents. Cross-linking in advanced polymers attracts grant money; morpholine’s sterics and electronics always nudging at higher performance. Downstream, developers experiment with its role as a co-catalyst in green transformations or solubilizer for poorly soluble reactants, turning a standard product into a launchpad for the next wave of sustainable chemistry.
Regulatory scientists surveyed both acute and long-term exposure effects of 2-Morpholinoethanol. Short-term inhalation or skin contact may cause irritation, and laboratory rodents exposed to high doses showed minor hepatotoxic changes. Studies in aquatic toxicity reveal moderate levels of concern, with synthesis plants monitoring discharge carefully to avoid ecological buildup. Chronic occupational exposure assessments point to the need for well-maintained ventilation and annual worker health checks. Available mutagenicity and carcinogenicity studies, as cataloged by ECHA, place this molecule below many aminated industrial intermediates on the hazard scale, though REACH registrations mandate annual dossier updates as new data arrives. Most regulatory authorities require handling precautions for manufacturing and warehouse employees but permit formulated end-products in topical, indirect food contact, or utility water applications.
Innovation teams keep tabs on 2-Morpholinoethanol as markets demand greener solvents and more flexible building blocks. The steady emergence of waterborne coatings and advanced surfactant systems props up demand, while regulatory scrutiny nudges process chemists toward cleaner production routes. Interest in recyclable catalysts and biocompatible polymers points toward modifications of this molecule, embedding morpholine’s profile in new functional hybrids. As chemical supply chains tighten and users seek reliable properties married to easier disposal, the appeal of a dual-function amine-alcohol stays strong. Cross-sector collaboration could bring 2-Morpholinoethanol from old-school corrosion inhibitor to star performer in the next generation of performance materials, provided its safety and supply remain well managed.
A bottle with a long name—2-Morpholinoethanol—first caught my eye in a university storeroom. It was labeled for use in “buffering and synthesis.” Back then, I couldn’t imagine how a single ingredient slid into so many areas: research, medicine, cosmetics, even coatings. Products like this rarely grab headlines, but they shape plenty of what we take for granted.
Chemistry classrooms and labs stock a lot of tried-and-true compounds, but very few bring versatility like this one. In the world of biochemistry, it’s used to help keep solutions stable. Many researchers prepare buffers with 2-Morpholinoethanol, keeping their pH steady during long experiments. Researchers depend on this, since pH swings can unravel days of careful work. Their trust isn’t misplaced: review articles and lab supply companies show thousands of uses each year.
Buffering solutions isn’t the only knack. This alcohol-amine helps chemists build more complex molecules. I’ve watched chemists turn to 2-Morpholinoethanol when tweaking the structure of pharmaceutical candidates. Small changes in synthesis matter—one extra carbon or nitrogen can change a drug’s behavior. During drug development, nimble chemistry means faster breakthroughs. That alone justifies its spot on any serious workbench.
Never underestimate how far a raw ingredient can travel. Chances are you’ll find 2-Morpholinoethanol in paints and coatings. It acts as a surfactant, breaking up bubbles and smoothing layers. Everyone loves a wall free from streaks; factories rely on reliable ingredients to meet expectations. There’s a simple lesson in this: the overlooked choices in industrial settings trickle into the look and durability of our homes and offices.
Digging into product labels brings another surprise: personal care products. Some skin creams and lotions use 2-Morpholinoethanol as a pH adjuster. It steps in when companies want something gentle and predictable. Safety regulators in Europe and the U.S. have set limits, and responsible brands stick to them. I’ve talked with chemists working in skincare; their comfort comes only after batches pass testing, matching regulatory rules for skin safety.
No chemical choice is perfect. Handling 2-Morpholinoethanol calls for gloves and goggles, not just caution taped on a wall. Exposure at the factory, especially in bulk, needs real training and attention. Public databases show irritation risk if used improperly, so safe handling deserves more than a passing glance. Mistakes happen rarely in large-scale labs, but small-scale users—side projects or DIYers—sometimes underestimate the hazards. Clear labeling and education could do even more.
Waste disposal creates another challenge. Regulators spell out how to cleanly get rid of unused product. Wastewater treatment plants see countless substances pass through each day. Industry and labs should follow guidelines down to the letter. Otherwise, trace chemicals build up in waterways, a problem we already see across the globe. Cleaner chemistry and improved waste tracking can chip away at this issue, making a difference on an even larger scale.
2-Morpholinoethanol isn’t famous, but its fingerprints turn up in science, industry, and the stuff we use at home. Its utility depends on well-trained hands, strict rules, and good habits. Scientists, factory workers, and product designers—together, they decide whether this useful chemical supports health and innovation or becomes a silent source of trouble. That balance deserves a sharper public eye and more open discussion every step of the way.
2-Morpholinoethanol pops up in chemical supply catalogs. Factory workers, lab staff, and researchers know it by its clear, syrupy look and light fishy smell. Many people outside those fields barely hear the name. That doesn’t mean folks shouldn’t care. Chemicals slip into daily routines more than most people think, whether through industrial pollution, accidental spills, or mishandling in small workshops. Understanding what’s in the air or on your skin matters, especially with chemicals like this.
This compound doesn’t live up to the infamy of strong acids or nerve agents, but it isn’t harmless. The safety data sheet for 2-Morpholinoethanol pegs it as an irritant, especially to the skin and eyes. Reports show red, itchy skin or burning eyes if protective gloves and safety goggles are skipped. It can sneak into airways during use in manufacturing, leading to coughing or sore throats. The fuss over irritation isn’t hype either — repeated exposure makes things worse, especially if spilled or handled in tight, poorly ventilated spaces.
Breathing a lot over time ramps up health risks further. Workers in textile finishing and other chemical workplaces share stories about stuffy noses or dry coughs after a day around the compound. Swallowing it is rare, but even a small accidental gulp leads to stomach pain, nausea, or more serious trouble. Too much on skin or accidental splashes into eyes send people to the medical tent with burns that take days to calm down.
Data comes mostly from workplace exposures and animal tests. The U.S. Environmental Protection Agency and similar offices label 2-Morpholinoethanol as a substance needing careful handling. The chemical’s structure means it can slip through skin and eventually affect other organs, not just the surface. Lab tests in mice and rats showed effects on liver and kidneys at higher doses. Poison control centers track cases where misuse or accidents occur, and health regulators set exposure limits for jobs that use this chemical a lot.
No one likes a pile of paperwork and training, but skimping on safety puts both health and business at risk. It pays to keep gloves, proper air flow, and protective goggles standard in any setting touching this chemical. Relying on “just a quick splash” thinking leads to rashes, days off work, or, in rare cases, hospital visits. People working in chemical plants or labs often get reminders about mixing up chemicals without a plan, just because the product label looks routine.
Better air circulation drops the risks from breathing in vapors. Good labeling stops curious hands from grabbing the wrong bottle. Locking up strong chemicals in closed cabinets keeps accidents with kids or pets away. Having antidotes or eye-wash stations on hand isn’t just regulation — it’s practical when things go sideways. It helps, too, when companies train the people who clean and throw away waste — not just scientists and management — on what to do in a spill. Risk vanishes fastest when everyone, from the newcomer to the boss, treats safety like part of the day, not an afterthought.
No matter the chemical, respect and knowledge add up to fewer accidents and healthier lives. 2-Morpholinoethanol brings risk with sloppy handling. With clear heads and straightforward practices, work stays safe and accidents drop.
2-Morpholinoethanol starts with a pretty simple formula: C6H13NO2. This means six carbons, thirteen hydrogens, one nitrogen, two oxygens—all packed together in a small chemical that finds its way into a surprising range of lab and industrial work. Its chemical structure sets it apart, blending features from two common chemical groups: it holds a morpholine ring (that’s a six-membered ring, with four carbon atoms, one oxygen, one nitrogen) connected through an ethanol side chain. The ethanol piece comes from a two-carbon group with an -OH at the end, which helps with its solubility in water and makes it handy for many reactions.
Looking more closely, the chemical structure for 2-Morpholinoethanol can be written as HOCH2CH2N(C2H4O)2. For someone who’s worked with organic chemicals, those morpholine rings always bring to mind versatility: you get a little bit of basicity from the nitrogen, but also the ability to dissolve in water or a solvent like alcohol. This combination creates a flexible molecule that plays nicely with both polar and non-polar substances. A real bonus in a chemical toolbox.
I’ve seen 2-Morpholinoethanol pop up most often in the lab among buffer solutions or as a starting point for synthesizing drugs or other compounds. In paint or coatings, folks might use it as a co-solvent that helps blend other ingredients. Its morpholine backbone attracts reactions with acids and acylating agents—plus, the –OH group opens up extra possibilities. For those formulating cleaning products, its solubility and mildness mean fewer worries about corrosion, which I’ve found is a sticking point for plenty of companies trying to move away from harsher chemicals.
From experience, it pays to treat 2-Morpholinoethanol with care. Its structure allows absorption through skin or mucous membranes. Not as toxic as some traditional solvents, but a splash or long exposure can still cause irritation or more lasting effects. The Centers for Disease Control and Prevention notes the importance of proper ventilation and gloves in workplaces using this molecule. Unlike strong ammonia or caustic solutions, the risks feel manageable, with reasonable safety guidelines. Still, there needs to be respect for its potential in mishandling.
Modern manufacturing looks at more than just effectiveness. Disposal and environmental fate for organic solvents now draw close attention. Morpholine derivatives like 2-Morpholinoethanol have shown biodegradability in certain tests, which scientists see as a step toward greener chemistry. Limitations still exist: widespread use could mean buildup if disposal practices lag behind production. Impact studies often urge switching to compounds that break down faster, or installing specialized treatment systems in factories that discharge wastewater. From what I’ve seen, the best results come from a shift in mindset—choosing such chemicals for key roles, not just out of habit, and committing to responsible handling all the way from storage through disposal.
Progress always relies on thoughtful decisions at each stage. In the case of 2-Morpholinoethanol, leveraging its solubility, stability, and ability to form buffers can kickstart a greener or more efficient workflow. Teams shouldn’t overlook a quick review of safer alternatives or potential upgrades to personal protective equipment. Open discussion with suppliers about sourcing and downstream impact also helps push for transparency and accountability.
I’ve worked in labs where chemical safety wasn’t just a box to check, but something people thought about every day. 2-Morpholinoethanol can look pretty harmless at first glance—a colorless liquid, maybe a mild scent if the bottle’s open too long. What most folks don’t see are the risks hiding under that plain appearance. Even before you unscrew the cap, things like skin and eye irritation are on the table, and no one wants a splash in the wrong place. That’s why handling and storage can’t turn into a rushed afterthought. If you’ve ever seen a spill turn serious fast, you probably feel the same.
Many chemicals, including this one, stay stable if you store them in a cool, dry space. It’s not enough to slide it onto any available shelf or back corner in a storeroom. Air, heat, and light can all nudge 2-Morpholinoethanol into breaking down or reacting in unpredictable ways. I’ve seen lab managers set up dedicated cabinets, away from acids and oxidizers, with every container capped tightly and clearly labeled. Flammable storage cabinets aren’t just showpieces; they can limit accidents and make emergency responses quicker. Safety data sheets often state to keep the chemical at a set temperature range—if a fridge is recommended, keep it there. Ignoring these details may seem harmless, until a bottle leaks or builds up enough vapor to pose a real hazard.
PPE gets its reputation for good reason. Gloves, goggles, and a lab coat form the first line of protection, and a face shield tops off the kit during transfers. It’s easy to get lazy about this when rushing, but I’ve seen what happens when someone thinks “just a little bit” is safe to handle bare-handed. Even a drop can cause burns or trigger a respiratory reaction. Good ventilation helps a ton—open windows don’t carry enough weight compared to working under a fume hood. It controls vapors and keeps exposure low. Pour carefully and use pumps or pipettes for transfers. Splashing isn’t just a mess; it’s a risk.
Spills or leaks shouldn’t sit. Small spills can get cleaned with absorbent pads, but then they go straight into a hazardous waste container—never tossed with regular trash. People sometimes shrug off reporting minor accidents, but every near miss matters. Quick cleanup and open communication stop little problems turning into big ones. Emergency showers and eyewash stations need regular checks, not just a sticker that shows they passed inspection last year.
Training sessions don’t have to be long, but they do need to cover real risks and show the right way to use every piece of equipment. I’ve found that people listen closer when trainers share real stories—how a lazy habit led to a trip to the ER, or how careful storage kept a spill contained. Regular check-ins help reinforce these habits. Maintaining up-to-date labels, reading safety data sheets, and practicing emergency procedures all feed into a culture that values human health and the well-being of everyone in the space. Working with chemicals like 2-Morpholinoethanol might feel routine, but safe practices set the foundation for everything else that happens in the workspace.
2-Morpholinoethanol is one of those chemicals that keeps surfacing in the lab, whether it's for testing solvents, adjusting pH in a formulation, or looking for compatibility in surfactants. It’s a clear, colorless liquid, sometimes mistaken for its relatives in the morpholine family, but it brings its own quirks to the table. Knowing how it behaves physically helps avoid surprises—because nothing good comes from a runaway reaction or a miscalculated distillation.
2-Morpholinoethanol boils at about 180-185°C. That high boiling point means it sticks around during heating, which works well for processes that require steady evaporation rates and control over volatile loss. I’ve seen it hold its ground in heated baths and reflux systems, unlike ethanol or acetone, which often vanish sooner than planned. Its melting point sits close to -1°C, so in most lab fridges this material stubbornly stays liquid. No sudden surprises or blockages in tubing when it gets a bit chilly.
This chemical feels slick—not quite as light as water, but not heavy like glycerol. Viscosity plays into how it mixes; spill a bit, and it runs, but doesn’t disappear in a flash. It’s completely miscible with water, so diluting or cleaning up takes less effort than dealing with oily organics. Its faint, amine-like odor reminds me it’s part of a family that demands good ventilation.
2-Morpholinoethanol has a density near 1.07 g/cm³ at room temperature. I never had to worry about phase separation in aqueous systems; it always disappeared right in—no floating, no sinking. From the shelf stability viewpoint, the compound doesn’t complain much. As long as the bottle stays sealed and out of direct sunlight, degradation isn’t something that creeps up overnight. Avoid storing it with strong acids, since the amine function understandably reacts, which is standard practice with amine-bearing organics.
It’s not the most hazardous chemical in the cupboard, but gloves come out every time. I remember a careless splash—skin tingled for hours. 2-Morpholinoethanol causes irritation, and good ventilation saves everyone a headache. Lessons learned: goggles, gloves, and, ideally, a splash screen during transfer. Some regulatory agencies point out that it's not classed as carcinogenic or acutely toxic, but nobody should get complacent around any amine alcohol.
In research or small-scale industry, properties like boiling point and solubility affect real world choices. If a chemical boils off too fast, the lab gets messy and the yields drop. Low melting points prevent clogs, especially in climate-controlled suites or during cold processing steps. High water miscibility makes life easier during dilution, mixing, and cleanup stops. Getting familiar with the physical quirks of 2-Morpholinoethanol helps keep things running smoothly—personally, I trust materials more when their behavior is predictable and manageable, especially under pressure.
Accurate info about physical properties comes straight from both analytical labs and years of consistent reporting in scientific literature. Merck Index entries, supplier safety sheets, and real-life testing in academic or industrial settings give the numbers everyone relies on, so there’s little room for dangerous guesswork. Relying on thorough handling protocols, clear labeling, and up-to-date training keeps accidents out of the storyline. The world of chemicals asks for respect, and knowing the quirks of each compound keeps both people and projects on track.
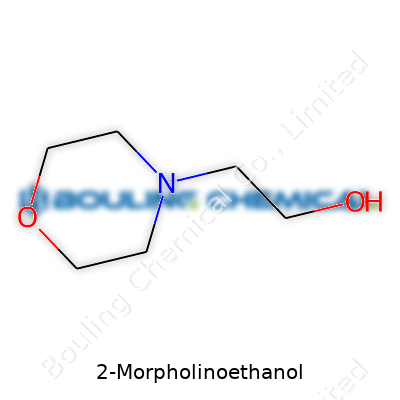
| Names | |
| Preferred IUPAC name | 2-(Morpholin-4-yl)ethan-1-ol |
| Other names |
N-(2-Hydroxyethyl)morpholine 2-(Morpholin-4-yl)ethanol Morpholine-2-ethanol |
| Pronunciation | /tuː mɔːˌfɔːlɪˈnoʊ iːˌθæ.nɒl/ |
| Identifiers | |
| CAS Number | 622-40-2 |
| 3D model (JSmol) | `3DModel:JSmol{"mol":"CCN(CCO)CO"} |
| Beilstein Reference | 1465066 |
| ChEBI | CHEBI:77743 |
| ChEMBL | CHEMBL183278 |
| ChemSpider | 68210 |
| DrugBank | DB08797 |
| ECHA InfoCard | 100.007.876 |
| EC Number | 2.2.7.1 |
| Gmelin Reference | 71596 |
| KEGG | C06230 |
| MeSH | D016624 |
| PubChem CID | 77145 |
| RTECS number | KK6825000 |
| UNII | 1X50A8A4CC |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C6H13NO2 |
| Molar mass | 133.18 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | amine-like |
| Density | 1.051 g/cm3 |
| Solubility in water | miscible |
| log P | -1.32 |
| Vapor pressure | 0.02 mmHg (20°C) |
| Acidity (pKa) | 8.08 |
| Basicity (pKb) | 5.55 |
| Magnetic susceptibility (χ) | -8.55×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.468 |
| Viscosity | 19 cP (25 °C) |
| Dipole moment | 1.25 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 252.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -464.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3861.6 kJ/mol |
| Hazards | |
| Main hazards | Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319 |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 93 °C |
| Autoignition temperature | 190°C |
| Lethal dose or concentration | LD50 Oral - rat - 4,395 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 5,120 mg/kg |
| NIOSH | WSH40200 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-Morpholinoethanol: "Not established |
| REL (Recommended) | REL (Recommended): 10 ppm |
| IDLH (Immediate danger) | 100 ppm |
| Related compounds | |
| Related compounds |
Morpholine Diethanolamine Aminoethanol |