Chemistry often makes its best advances through small, steady steps, and 2-Morpholinoethanesulphonic acid, or MES, showcases this path. The compound emerged during the surge in buffer development through the 1960s and 70s, led by researchers needing stable, non-interfering agents for controlling pH in biological work. This period saw researchers like Norman Good and his peers wrestling with rapid changes in scientific equipment and techniques. They looked for buffering solutions that avoided enzymes, tests, or cell systems from drifting out of the optimal range. MES, by delivering predictable buffering capacity around pH 6.1, slotted in among these resources. Its popularity kept rising as biologists, chemists, and pharmaceutical scientists all sought ways to run repeatable and clean experiments.
The official IUPAC name for MES is 2-(N-morpholino)ethanesulfonic acid. Scientists often shorthand its name just to MES. This white, crystalline powder dissolves easily in water, producing clear solutions. Labs count on its low ultraviolet absorption, so photometric tests go off without odd background noise. Because it rarely interacts with most metals and biological molecules, MES finds a home in protein work, enzyme research, and cell culture. It stands out for not crossing cell membranes. Unlike some early buffers, you don’t end up disrupting internal cell chemistry.
MES has a molecular formula of C6H13NO4S and a molar mass of about 195.24 grams per mole. The melting point sits near 300°C with decomposition, underscoring its thermal stability in most settings. In water, the solubility climbs past 100 grams per liter at room temperature, delivering concentrated stock solutions for busy labs. The acid’s pKa at 25°C measures roughly 6.1, which places it right in the sweet spot for buffering mild acids and bases—often needed when working with mammalian cells or delicate enzymes. MES powders tend to flow easily, and solutions remain colorless. Its stability under autoclaving saves time during media or buffer prep, since users can sterilize without degradation.
Vendors label MES by its purity (frequently ≥99%), along with any traces of heavy metals or moisture. For biochemical work, pharma and academic labs demand lots with low endotoxin and bioburden counts. Product labels highlight batch numbers, shelf life, storage conditions, and recommended container types. The designated hazard warnings deserve attention. In my own experience, labeling matters most when multiple staff share a central reagent supply, and stock is stored for months. Double-checking batch data on the bottle proves crucial for troubleshooting sudden changes in assay performance.
Manufacturing MES usually starts with ethylene oxide or ethylene chlorohydrin and morpholine, leading to an intermediate which reacts with sodium bisulfite. This chemical sequence needs careful temperature and pH controls. After reaction, the solution gets stripped of salts and other byproducts, then the acid is crystallized, rinsed, and dried. Finished product testing checks solid state purity, confirmed by spectroscopic methods or chromatography. Consistency and trace impurity testing become central quality points, since the tiniest change may derail a delicate experiment.
MES doesn’t participate directly in many reactions unless pushed under strong conditions. Its morpholine ring resists breaking except under strong acids or bases. Labs sometimes tweak MES’ sodium or potassium salt form, depending on the target experiment. You’ll rarely find the compound in oxidation, reduction, or substitution reactions outside these niche uses. The molecule’s structure, with a sulfonic acid group linked to a heterocyclic ring, mostly blocks it from reacting with metal ions or most organic reagents under typical lab setups.
2-Morpholinoethanesulphonic acid appears under many aliases: MES, MES acid, and 2-(N-morpholino)ethanesulfonic acid. Trade catalogs announce it by its CAS number: 4432-31-9. Most major suppliers carry at least two qualities—biotech grade and analytical grade—matching them to either research or clinical use. A researcher may find the labeling “MES monohydrate” or “MES free acid” depending on water content.
Handling MES doesn’t resemble classic laboratory hazards but shouldn’t lull anyone. Dry powder can bother the lungs or eyes. Skin contact rarely leads to trouble, though gloves protect from chronic irritation. Safety data sheets (SDS) spell out cleanup and emergency procedures, as MES poses little threat of combustion or sudden reactivity. Storage in cool, dry places extends shelf life, since water uptake changes the powder’s mass and purity. I remember labs where students forgot to seal bottles, only to return to sticky, compacted cakes—small mistakes, but ones that can throw off years of buffer recipes. Good habits, clear labeling, and proper storage remain fundamental.
MES anchors modern biology and biochemistry labs. Biologists working in cell and tissue culture rely on it to keep environmental pH rock-steady. Protein chemists choose MES when working close to neutrality, notably with enzymes or antibodies sensitive to other amine-based buffers. Its negligible metal-binding stops interference with enzyme cofactors or crucial ionic signaling. Water test engineers lean on MES’ clear solutions to avoid signal drift in photometric measurements. Diagnostic kit makers and pharmaceutical developers prize MES for its lack of inhibition in enzyme-linked immunoassays. Years of personal lab work have demonstrated the difference between a clean MES buffer and a hastily-made phosphate one—many experiments demand the right buffer to interpret results with confidence.
MES didn’t freeze in time after its early invention. Scientists keep testing its performance in odd conditions, mixing it with salts, surfactants, and organic solvents to measure interactions. Manufacturing teams strive to cut trace contaminants, which can skew protein stability or enzyme reactions. MES regularly gets compared to its chemical cousins (PIPES, HEPES, and MOPS), with new publications weighing their pros and cons for cell biology, clinical assays, and fermentation. Modern labs even look for MES analogs with sharper buffering or different solubility, trying to match ever-more-specialized experiments. Over the decades, published protocols for MES-buffered solutions number in the thousands, touching fields ranging from crop science to synthetic biology.
MES doesn’t appear acutely dangerous at reasonable concentrations used in the lab. Animal studies point to low oral and dermal toxicity. Cell viability tests show little metabolic disruption. But data gaps linger—long-term exposure or high doses might lead to subtle health effects. Environmental chemists keep tabs on MES’ breakdown if dumped in wastewater, given its stability against natural degradation. For most workers, respecting general lab hygiene suffices, although repeated spills and careless handling could lead to respiratory or minor allergic issues. Tracking lab safety reports and vendor updates helps clarify if new risks emerge over time.
MES has reached a stage where it’s unlikely to vanish from scientific labs. Its low cost, performance, and track record support ongoing use in buffer recipes, diagnostic kits, and pharmaceutical manufacturing. As experimental sensitivity improves, demand grows for ultrapure grades and careful handling. Research may reveal new limits or rare pitfalls, pressing scientists to keep testing for minute artifacts. Industrial applications, like fermentation or process control, increasingly require compounds that don’t cross-react in complex mixes; MES holds an edge in these spaces. If environmental rules or health standards evolve, chemistry firms may adjust their processes to guarantee safety, purity, and disposal compliance. For anyone charting the buffer field, MES delivers a rare blend of reliability and scientific flexibility.
Ask anyone who’s stepped foot in a life sciences lab, and chances are they’ve run across 2-Morpholinoethanesulphonic Acid at some point. Most folks just call it MES. Its main role isn’t fancy; MES buffers solutions, quite often in biology and chemistry research. If a scientist tries to grow cells or study enzymes, the pH of the environment has to stay steady. Enzymes really care about the conditions they work in, and changing pH can wreck results or kill cells off. MES works well for this, holding the pH in a narrow window, especially between 5.5 and 7.0.
MES makes itself useful because it doesn’t interfere much with biological reactions. I’ve watched researchers fill their benches with glassware and bottles labeled “MES buffer,” running experiments on proteins, bacteria, and even plant cells. The fact is, MES doesn’t mess up the data. Other buffering chemicals—phosphate or Tris—sometimes react with what you’re studying. MES just sits quietly in the background, keeping things steady. That characteristic has saved countless biology grad students from having to rerun experiments.
Reliable science depends on reproducibility. If conditions change even a bit during an experiment, results get fuzzy. MES doesn’t just stabilize pH; it helps labs around the world compare their findings. One group’s experiment in Singapore can match a group’s work in Boston, all because they used a buffer like MES. The scientific literature backs up this point. Journal articles from as far back as the 1960s report the value of using “Good’s buffers,” including MES, for reproducible results.
The need for quality chemicals has grown as biotech and pharmaceutical companies ramp up their work under strict standards. I’ve sat in meetings where regulatory staff stress the importance of using pure, certified chemicals. MES comes in grades that meet these tough requirements. High purity means less contamination risk in sensitive experiments, from vaccine research to drug testing. Regulatory agencies watch every step, so scientists can trace the source and handling of each batch.
MES isn’t the cheapest buffer out there. Not every lab has unlimited funds, especially in countries where research money doesn’t stretch far. Sometimes, basic science slows down because supplies run short or budgets dry up. There’s a need for manufacturers to keep the price reasonable and make sure that labs, even smaller ones in developing regions, have reliable sources. Open, transparent supply chains protect research from counterfeit or mishandled chemicals.
Handling chemicals of any sort demands care, even those with a good safety record. MES isn’t considered dangerous, but accidents happen if safety gets ignored. Simple habits, like using gloves and goggles and keeping good records, spare people from trouble. New lab staff benefit from training that drives these habits home. Creating a safety-focused culture helps everyone work more confidently and minimizes risk, protecting people and projects alike.
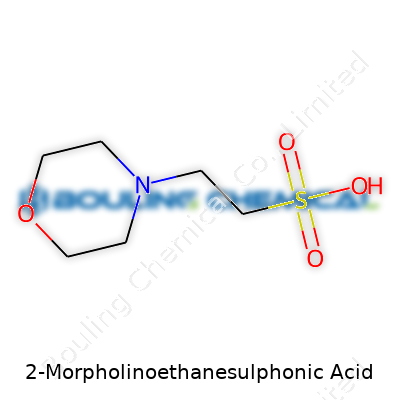
2-Morpholinoethanesulphonic Acid goes by the everyday name MES. Its molecular formula is C6H13NO4S, with a molecular weight of 195.24 g/mol. MES fills a steady role in labs focused on biochemical and molecular biology research. The accuracy of this information isn’t only trivia for student chemists or lab technicians. Calculating concentrations, preparing buffers, and confirming purity all hang on knowing those numbers precisely. Get the formula wrong, and downstream processes can derail, wasting time and precious resources.
From years of prepping lab solutions, I’ve seen what happens when even minor mistakes sneak into fundamental calculations. Drop too much or too little MES powder into your solution because of a slip in math or a flawed reference and watch results take a hit. Often, experienced teams double-check molecular weights and formulas before weighing anything, a practice that keeps projects and grant funding on track.
Fact-checking details like this comes up every semester in university labs. Relying on verified sources—published journals, Sigma-Aldrich, or Merck manuals, for example—pays back. Sigma-Aldrich describes MES as a commonly used buffering agent favoring pH ranges 6.0 to 7.4, crucial information in protein and DNA studies. Keeping this data handy isn’t just academic, it's a real prevention tool against botched experiments and wasted budgets.
MES serves environments where biological molecules need a stable pH. Running electrophoresis gels or culturing sensitive cell lines? MES ensures the system doesn’t shift too far acidic or alkaline. That reliability allows repeatable results and data integrity. One researcher I worked alongside used the wrong buffer for a protein purification run and watched weeks of effort melt away. Accurate chemical information is worth more than gold in that setting.
Careful labeling and documentation is the backbone of lab safety and quality assurance. CAS number 4432-31-9 assigns MES a spot in regulatory filings and supply orders, making sure everyone, from undergrad to principal investigator, talks about the same compound. Cross-checking batch certificates for molecular weight can also spot impurities or degrade from poor storage, protecting against contamination in sensitive projects.
Teaching young scientists the significance of getting the molecular formula and weight right is more than a technical requirement. It grows attention to detail, accountability, and professional pride. In clinical labs, I’ve watched how one misplaced decimal point triggered confusion across entire workflow chains. Documenting every step, routinely reviewing source data, and using digital tools all cut down on errors while boosting transparency and reproducibility.
Quality scientific investigation depends on sticking with details that some might consider trivial. Accuracy with MES reflects a broader habit of care and diligence. Supporting teams with up-to-date chemical databases, fostering open discussion around preparation steps, and encouraging verification before any solution leaves the balance room can prevent setbacks and elevate research outcomes. The difference between discovery and dead ends often rests on the humble digits behind names like 2-Morpholinoethanesulphonic Acid: C6H13NO4S, 195.24 g/mol.
Anyone who’s pipetted endless microcentrifuge tubes knows how much depends on having the right buffer. Get it wrong, and cells stop growing, enzymes lose punch, or western blots ghost out. Out of the shelf’s rainbow of possible buffer chemicals, 2-Morpholinoethanesulphonic Acid—most folks call it MES—sits on a lot of benches. But why do researchers keep picking MES buffer, and do its strengths really suit the mess of modern biological jobs?
MES covers a pH range from about 5.5 to 6.7. That zone hits right in the sweet spot for culturing plant cells, keeping certain enzymes snug, and handling proteins that fuss anywhere too acidic or too basic. In my own work with bacterial growth experiments, drifting out of the optimal pH zone left cultures sluggish or totally dead. MES holds the line against sudden shifts, which keeps those experiments running smoothly and gives data some consistency across batches. Few things kill reproducibility faster than a creeping pH shift.
Sure, classic phosphate buffers cost less and you can find them in every catalog. The trouble is, phosphate can tie up with calcium and magnesium—wrecking sensitive experiments like those with membrane proteins, DNA, or some metal-dependent enzymes. Years ago, a team in our lab got wild data swings until we ditched phosphate and switched to MES. Right away, our calcium measurements sharpened up overnight.
MES doesn’t show up in many biochemical interference charts. That means it reacts with fewer reagents, doesn’t slow down enzyme activity, and keeps your sample proteins happier. I have seen improvements in immunoassays and ELISAs once we swapped out harsher buffers for MES, because its sulfonic acid group doesn’t poke at protein structure or clog up antibody action.
Years of running experiments taught me never to pick a buffer just because it’s popular. Even top brands sometimes sell batches packed with impurities—formaldehyde in particular can haunt MES lots if synthesis skips careful controls. Poor-quality MES doesn’t just mess up sensitive chromogenic assays, it can set up background signals that make it impossible to draw clear results from data. Always check for reputable suppliers who publish third-party purity analyses and batch traceability.
MES isn’t the rock-bottom cheapest, but it isn’t in the ultra-pricey club either. For most labs, budget strains show up more from the number of failed, repeated experiments than the chemical cost itself. Unlike some buffers, MES doesn’t emit strong odors, isn’t volatile, and doesn’t carry severe toxicity warnings. That may not sound flashy on paper, but it matters when prepping liters of solution a week. At the end of the day, safe disposal matters for everyone. MES biodegrades more easily than Tris or phosphate, so the environmental load doesn’t pile up as heavily around university outflows or hospital labs.
Biological experiments keep getting sharper, and the buffers of yesterday don’t always make the cut for newer, more demanding protocols. The versatility, low chemical noise, and strong pH holding of MES make it a top choice across research areas where pH precision and chemical compatibility matter. In decades of real-world lab work, I’ve seen MES help avoid ruined samples, wrong data, and costly reruns—enough to keep it close at hand for any run that really counts.
When labs store chemical reagents, routine and care make a big difference. For a buffer like 2-Morpholinoethanesulphonic Acid, most folks call it MES, storage isn't glamorous but it’s critical. I’ll never forget the mad scramble to find the source of a failed experiment in my postgrad days. It wasn’t the protocol—it was the buffer. The bottle had lived through more temperature swings than anyone realized, and contamination crept in. It happens quietly, then suddenly your data is off, and all eyes turn to the recycled container in the fridge. Safe storage takes planning and discipline.
MES holds up well dry, so shelf storage serves most labs just fine. Keeping it dry and sealed keeps moisture and other contaminants out. Most suppliers recommend storing it at room temperature, in a tightly closed container, away from direct sunlight and any sort of high humidity. This matches what I’ve seen in biochemistry labs, where a dry cabinet or drawer does the job. The aim is to keep the powder clump-free and stop any subtle breakdown that could shift your buffer range. Water creeps up on open bottles, especially in coastal towns or buildings without great HVAC.
Exposure to air isn’t innocent. Chemicals pull moisture from the room every time someone opens the lid. Before long, humidity can lead to clumping, which throws off weighing and dosing. Some colleagues go the extra mile. They use silica gel packs in the same bin as their buffer stocks, just for an extra layer of dryness. If you have a desiccator, all the better—it’s a simple fix with long-term payoff, especially after you’ve seen how moisture can trigger caking in some buffers.
Once you start making MES solutions, that’s where things get trickier. Once dissolved, MES becomes more vulnerable. Bacteria love neutral pH and a ready-made nutrient bath. For solutions, most researchers recommend using sterilized water, then filtering through a 0.22-micron filter for jobs where sterility matters. It’s not overkill. Once contaminated, MES solutions can breed unwanted bugs fast, and visible cloudiness spells trouble. I always mark solution bottles clearly, track opening dates, and store them at 2-8 °C. Fridge space is tight, but it shields the solution from microbial growth and chemical breakdown.
Chemicals like MES last much longer if people don’t double dip or touch powder with damp tools. Always use a clean, dry spatula, and reseal bottles tightly. If possible, get smaller bottles instead of massive tubs, unless your workflow truly burns through MES weekly. Opened containers get exposed more, and old stock can betray your results months later.
Safety isn’t about one magic step. It’s a rhythm. Routine labeling, first-in-first-out inventory, and regular checks on expiry dates help prevent surprises. Keep a log of storage and use. Smart storage doesn’t just protect the chemical; it protects your work, your budget, and everyone who counts on reliable results. Real focus on how and where you keep MES pays off far more than any troubleshooting down the road for ruined buffers or wasted time.
2-Morpholinoethanesulphonic Acid shows up in research as a useful buffer, especially in biological work. This chemical has the ability to keep the pH steady, holding experiments on solid ground. People use it every day in labs focused on molecular biology, biochemistry, and cell culture. Though the name sounds intimidating, not every complex molecule brings danger to the bench.
Safety matters in the lab more than anything else. Years working in research convinced me that no chemical deserves blind trust. Hazardous chemicals hurt people, but so do careless moments. For 2-Morpholinoethanesulphonic Acid, safety sheets and hazard data settle most fears. Most suppliers label it as a substance that triggers eye and skin irritation. Some people may get a rash or start coughing if they breathe in dust. Handling it calls for gloves, lab coats, and eye protection. I used it countless times; never saw it eat through a glove or fog up a face shield.
This chemical lacks strong toxicity. Swallowing or breathing large amounts would stir up trouble, like headaches, stomach aches, and throat irritation. But 2-Morpholinoethanesulphonic Acid doesn’t fall in the same group as real dangers such as cyanide or strong acids. There’s not much evidence showing cancer risks, long-term damage, or genetic harm from this buffer. It will not explode, catch fire easily, or burn without serious help from other materials. Storing it in a cool, dry spot—sealed tight—does the job.
Caring for the environment counts just as much as health. Scientists learned to handle waste with respect. 2-Morpholinoethanesulphonic Acid doesn’t have a flashing symbol on any hazardous waste chart, but that’s no excuse for pouring leftovers down the sink. Small amounts in diluted form probably won’t wreck the ecosystem, yet a habit of dumping unknowns does nobody any favors. Good lab habits mean collecting waste in proper containers and sending it off with regular chemical disposal. This keeps labs tidy and the local water supply safe.
Fresh technicians sometimes look at a long name like 2-Morpholinoethanesulphonic Acid and freeze up. I always recommend reading the safety data before working with it, not because it’s especially scary, but to get in the habit of respecting every chemical. Companies train their people to label bottles, wipe up spills, wash hands, and never eat or drink at the bench. Knowing the basic risks helps new staff avoid surprises. In practice, this chemical rarely makes headlines for injuries or environmental accidents. It’s far more likely for mishaps to happen from mixing it with the wrong chemical, so attention and basic sense make the biggest difference.
Wear gloves, tie back long hair, and use goggles. Store the powder in a sealed jar, away from food, drinks, and strong bases or acids. Make sure spills get cleaned up right away—nobody likes crusty buffer powder tracked around the lab. Dispose of waste through the regular chemical waste program. Treat every chemical with a mix of caution and respect, no matter how harmless the safety data may seem. Training, teamwork, and a tidy workspace knock out most risks before they ever turn into a problem.
2-Morpholinoethanesulphonic Acid helps research run smoothly and safely. My own work taught me to avoid shortcuts and set an example for others. Giving a little extra thought to handling, storage, and waste pays off in fewer accidents and a safer place to work.
| Names | |
| Preferred IUPAC name | 4-Morpholineethanesulfonic acid |
| Other names |
MES 2-Morpholinoethanesulfonic acid 4-Morpholineethanesulfonic acid Ethanesulfonic acid, 2-(4-morpholinyl)- |
| Pronunciation | /tuː-mɔːrˌfɪl.oʊˌiː.θeɪn.səˈfɒn.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 4432-31-9 |
| Beilstein Reference | 1720295 |
| ChEBI | CHEBI:39099 |
| ChEMBL | CHEMBL254026 |
| ChemSpider | 2056 |
| DrugBank | DB03744 |
| ECHA InfoCard | 100.018.686 |
| EC Number | EC 252-162-9 |
| Gmelin Reference | 107211 |
| KEGG | C00544 |
| MeSH | D018754 |
| PubChem CID | 10733 |
| RTECS number | MP8050000 |
| UNII | C2BFX949V9 |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C6H13NO4S |
| Molar mass | 207.247 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.064 g/cm³ |
| Solubility in water | soluble |
| log P | -4.0 |
| Vapor pressure | <0.01 hPa (20°C)> |
| Acidity (pKa) | 9.1 |
| Basicity (pKb) | 5.6 |
| Magnetic susceptibility (χ) | Negligible |
| Refractive index (nD) | 1.484 |
| Viscosity | Viscous liquid |
| Dipole moment | 6.44 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 324.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -526.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2061 kJ/mol |
| Pharmacology | |
| ATC code | Not assigned |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07 Warning |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | Precautionary statements: P264, P270, P280, P301+P312, P305+P351+P338, P330, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 100°C |
| Autoignition temperature | Autoignition temperature: 410°C |
| Lethal dose or concentration | Lethal Dose (LD50) Oral Rat: > 5,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 5,450 mg/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 2 mg/m³ |
| Related compounds | |
| Related compounds |
Morpholine MES sodium salt HEPES MOPS PIPES |