Long before 2-Methylthio Pyrazine popped up in glossy catalogs for flavor houses or scientific suppliers, food chemists scoured roasted peanuts and coffee beans, looking for those missing puzzle pieces that gave snacks and drinks their crave-worthy, warm aromas. Early researchers noticed pyrazines popping up in everything from cocoa to grilled meats, tagging them as key drivers in the “baked” and “toasted” sensory notes we know so well. Back in the mid-20th century, as analytical techniques started getting sharper, scientists managed to isolate these molecules from complex natural mixtures. Strong curiosity around how changing a methyl or thiol group could tip the aroma profile drove folks in both academia and industry to synthesize derivatives just like 2-Methylthio Pyrazine—hoping these would precisely recreate the tempting smells of roasted nuts or chocolate bars. The shift from discovery in nature to bench-scale synthesis didn’t take long, given the food industry’s hunger for intense, repeatable flavors.
2-Methylthio Pyrazine belongs to the pyrazine club, a class of nitrogen-containing rings you’ll find in hundreds of foods after roasting, baking, or fermenting them. This particular molecule carries a distinct, nutty, and roasted odor with just a touch of earthiness, something that can plunge you into memories of fresh coffee or just-popped popcorn. Food scientists use it to sharpen or recapture that fresh-roasted taste in everything from breakfast cereal to savory snack coatings. Suppliers usually ship it as a concentrated liquid or sometimes as a diluted solution to help with handling, keeping it locked in tightly sealed, light-resistant bottles—nobody wants even a whiff to escape.
In the lab, you’ll notice 2-Methylthio Pyrazine as a colorless to slightly yellowish liquid with a strong, punchy odor. It weighs in with a molecular formula of C5H6N2S and a molecular weight of about 126 grams per mole. It won’t dissolve well in water but finds its groove in organic solvents, making it handy for food technologists working with oily or fat-rich recipes. With a boiling point around 190°C and decent thermal stability, it doesn’t lose its oomph during most food processing stages—crucial for high-temperature applications like baked snacks or instant coffee flavoring.
Buy 2-Methylthio Pyrazine from reputable suppliers, and you’ll spot purity indicators, GC-MS retention times, and batch traceability on each label. Food industry guidelines often peg purity above 96% to avoid harsh, off-flavors, with tightly monitored contaminant thresholds for things like residual solvents or heavy metals. Regulatory codes across jurisdictions usually require clear, unambiguous chemical names, CAS numbers—specifically, CAS 28523-86-6—and hazard identifiers where appropriate.
In a synthetic lab, chemists whip up 2-Methylthio Pyrazine by starting from pyrazine or its substituted derivatives. One common route involves introducing a methylthio group through a nucleophilic substitution—usually slapping a methylthio moiety onto a bromopyrazine skeleton using a methylthiolate salt. It pays to run the reactions under carefully controlled conditions—anhydrous solvents and protective atmospheres—since both pyrazines and sulfur-based compounds have their finicky moments. Purification tends to involve a series of distillations and vacuum treatments, ensuring the end product isn't tainted by sulfurous byproducts or unreacted starting materials.
Once you have a stash of 2-Methylthio Pyrazine, you can take the chemistry further. It’s possible to tweak the molecule by altering the position or number of methyl or thio groups, producing derivatives with unique flavor notes—deeper, greener, or more “grilled” than the original. Sometimes, researchers test oxidation routes to see if swapping the methylthio for a sulfoxide or sulfone kicks out new flavors or stability attributes. Being a nitrogen- and sulfur-rich compound, 2-Methylthio Pyrazine doesn’t shy away from tautomeric shifts or energetic redox partners—making it a curious playground for organic chemists.
Widely supplied as 2-methylthio-3-pyrazine or just 2-methylthiopyrazine, this aroma chemical also appears under names like 2-(Methylthio)pyrazine, NS-2333, and sometimes Fema No. 3463 on product sheets for flavorists. Trade catalogs love to dress it up with proprietary marks or batch designations, but the core structure stays the same.
Handling pyrazines means staying alert. That rich aroma can feel overpowering if bottles aren't sealed or a drop spills on your lab coat. Standard practice calls for gloves, eye protection, and always some kind of local exhaust ventilation—nobody enjoys a lingering sulfur hit in the air. Safety data sheets for 2-Methylthio Pyrazine warn against swallowing, skin absorption, or breathing high concentrations, though its acute toxicity is lower than many industrial chemicals. Regulatory agencies like the FDA, EFSA, and JECFA set firm use limits in foods, balancing flavor impact with consumer safety. In a modern flavor lab, spill kits and clear documentation support both daily operations and emergency plans.
Application for 2-Methylthio Pyrazine runs wide, mostly anchored in the world of food and beverage aromas. I’ve seen it in action boosting roasted nut flavors in breakfast cereals, fine-tuning the chocolatey undertones in dessert coatings, and even lending a backnote to certain types of soups or bouillons. Beyond the plate, it appears in specialty fragrances to mimic the comforting smells of roasted grains, a trick perfumers sometimes use for nostalgia-driven scents. Pet food manufacturers, chasing after more authentic “grilled” character, occasionally deploy it as well. Strict inclusion rates usually hover in the parts-per-billion range, because even a trace too much can swing from appetizing to overwhelming.
R&D labs love 2-Methylthio Pyrazine for the same reason chefs chase the perfect sear—it brings depth. Researchers keep experimenting with blends, pairing it with other pyrazines or related sulfur compounds to nail regional flavor signatures, such as the toasted notes in Southeast Asian coffee or the special edge in European malts. Work is ongoing to create “nature-identical” versions by biocatalytic methods, letting engineered microbes turn out the molecule from simpler sugars or amino acids. These approaches promise increased sustainability and cost savings, plus a better marketing story for “natural” food labels. Analytical chemists are sharpening tools for detecting residues at ever-lower levels, ensuring traceability and keeping final foods within safe exposure windows.
Toxicologists haven’t flagged 2-Methylthio Pyrazine as an acutely hazardous agent—it doesn’t rack up the danger scores of plenty other industrial chemicals. But nothing in this world comes risk-free. High-dose exposures in animal studies show minor liver enzyme elevations or occasional digestive upset, but the concentrations involved far exceed anything you’d eat through regular food. Regulatory reviews by JECFA and the like limit average dietary intake with a solid margin, based on chronic toxicity and mutagenicity screens. Newer generations of cell-culture assays help round out the picture, scanning for subtle gene expression changes or oxidative stress, though findings consistently reinforce the relatively low-risk profile at food-dosed levels.
Interest in 2-Methylthio Pyrazine keeps growing, not just for the food world but in the toolkit for “clean label” innovation and sustainable synthesis. As flavor companies look to build portfolios that echo natural complexity without side-stepping regulations, more attention lands on fermentation or enzyme-driven production. Advances in metabolic engineering could soon see microbes spitting out this molecule from basic feedstocks—cutting down waste and moving away from fossil-derived inputs. Regulatory landscapes evolve, so researchers keep refining risk evaluation tools and expanding databases on how trace levels move through typical diets. As consumers chase bolder, more authentic eating experiences, the demand for aroma molecules that deliver unmistakable roasted or nutty notes keeps pace—making 2-Methylthio Pyrazine not just a tool of the present, but an ingredient with staying power.
2-Methylthio pyrazine sounds like something you’d find in a lab behind a row of locked cabinets, but it pops up in the middle of your everyday life. Anyone who has opened up a bag of roasted coffee or broken a piece of freshly toasted bread has unknowingly come across compounds like this one. Scientists grouped it with a class called pyrazines, which plants and roasting processes use to crank out their signature smells and tastes.
I’ve walked into bakeries at dawn just to get that overwhelming wave of nutty, almost earthy warmth, the kind you can’t mimic with a candle. It turns out, food chemists figured out that even a dash of 2-methylthio pyrazine goes a long way in shaping those punchy, roasted, or savory notes people swear by in coffee, cereals, and chocolate. In my own kitchen, simmering onions or garlic fills the room with a backbone scent—not that different from what big food companies chase on a massive scale. They add minute quantities of this compound to snack chips, nuts, or even soups, trying to hit the bullseye of “homemade” for the customer.
I remember a time at a food expo when a rep from the flavor industry described these compounds as “magic dust.” He wasn’t far off. 2-Methylthio pyrazine lets processed foods stand shoulder to shoulder with slow-simmered dishes, at least as far as the nose and tongue can tell. It’s a backbone for flavors labeled as roasted, nutty, or meaty. Potato chips, for example, eaters often wonder why the “plain” kind tastes better from one brand over another. Odds are, some of those batch differences come down to tweaks with molecules like 2-methylthio pyrazine.
Big food isn’t just after flavor, it’s chasing consistency—a few micrograms here or there can create the unmissable, comforting taste of “fresh from the oven” or “just roasted,” and that helps lure repeat buyers. Looking at regulations, the FDA in the U.S. marks this compound as generally recognized as safe (GRAS) at the tiny levels used for these food purposes.
Stepping outside food, this molecule finds its way into perfume laboratories too. Its nutty edge helps craft fragrances that aim for a toasty or cozy feel, something that lingers but doesn’t scream artificial. I’ve tried to track down that mysterious whiff in some woody, gourmand men’s colognes—it’s no accident. Perfume houses grab onto everything that reminds us of warmth or home, so 2-methylthio pyrazine shows up in small doses here as well.
What sticks out to me is the sheer reach of this nearly invisible ingredient. It can help cover up off-odors in consumer goods—cleaners, even pet food. It’s the kind of behind-the-scenes touch that rarely gets any spotlight outside a chemist’s forum, but it shapes the experience for millions, every snack or sniff at a time.
Some folks worry about unknown things hiding under catch-all terms like “natural flavors” or “artificial flavors” on package labels. From what I’ve read and experienced in the industry, these molecules, used in vanishingly small doses, pass through layers of safety checks. Still, the wider conversation about processed ingredients remains valid. People want to know what’s going into their food—not just if it’s safe, but why it’s there in the first place.
One fix starts with clearer labeling and more open dialogue from companies. If folks understand what each flavoring does and why it’s used, a lot of suspicion melts away. Transparency helps, and so does education—there’s no harm in wanting both flavor and peace of mind.
Every time I wander through a grocery store aisle, and spot a label filled with scientific-sounding ingredients, I can't help but wonder what they actually do in my food. Names like 2-Methylthio Pyrazine barely register for most shoppers. In reality, these compounds play a central role in building flavors in snack foods, sauces, and everything fast-casual. Their presence shapes whether you get that toasted or nutty undertone in a favorite product. So, the safety issue isn’t something only chemists should pay attention to — it sits squarely in our daily meals.
This compound belongs to a family of pyrazines popular in food technology for creating roasted, earthy notes that make processed and packaged goods taste a bit more like real cooking. It crops up in things like roasted coffee, baked products, and even some savory seasonings. The fact that it doesn’t occur as a whole food but rather as a manufactured aroma chemical can seem worrying to some, especially since ingredient labels often hide these flavor enhancers under generic titles.
If you dig into official statements, food regulators such as the FDA and Europe’s EFSA have given 2-Methylthio Pyrazine the thumbs up as a food flavoring. The flavor industry has been using it for years, and they haven’t reported major incidents tied to its legal use. Animal studies using realistic intake levels haven’t shown signs of toxicity. But, that’s based on current knowledge and use patterns. A vast amount of food science relies on large studies showing that minuscule amounts added to food pose little or no risk, and regulators periodically review new data when it pops up.
People don’t just want official “generally recognized as safe” tags; many want a clear reason to trust these chemicals sitting in their food. I get where this comes from — scare stories about additives seem to crop up every year. Knowing a compound doesn’t build up in the body or persist in food beyond its intended purpose goes a long way. Transparency is key here. If manufacturers explained not just safety, but also why these flavorings are picked over real ingredients, a little more trust would likely follow. As a cook at home, nothing beats real ingredients, but in the world of factory-made foods, shelf-life matters.
Consumer choices still shape what appears on shelves, and pushing for clearer labeling probably nudges companies to be honest about what goes into snacks and seasonings. Supporting further studies — not only focused on direct toxicity, but long-term dietary exposure — would also help build a robust body of evidence. In the short run, checking for updates from food safety groups and voting with your wallet can steer the conversation.
One thing is for sure: most of us crave flavor, and compounds like 2-Methylthio Pyrazine help achieve it. Whether that fits into your idea of healthy eating or not, paying attention and asking questions is part of eating well in a modern world where few meals are truly “from scratch.”
Everyday foods often get their character from a handful of strong-smelling, low-dosage chemicals. 2-Methylthio pyrazine takes its spot in that lineup, especially in products with nutty, roasted, and savory notes. Many people walk past the supermarket shelves unaware this compound helps make a potato chip taste more like a real baked potato. Its flavor punch comes from a molecular structure packed with roasted and sulfurous tones, keeping formulators coming back to it for authentic notes in flavors ranging from coffee to cocoa to snack blends.
Sitting in my first R&D lab years ago, I watched flavorists treat these pyrazines with respect—mostly because a slip of the hand could send a snack formula right into “burnt rubber” territory. Nobody wants that. Dropping in even a few milligrams too much in the vat set off the smoke alarms and got us back to square one. Every synthetic flavor compound behaves this way, but pyrazines feel especially touchy because of their potency and the way they mesh (or clash) with other taste notes.
Most flavor houses—and the published technical literature—recommend staying low with 2-methylthio pyrazine. Typical inclusion ranges run about 1–10 parts per million (ppm) in finished food or beverage products. That means, for a batch of chips meant for the U.S. market, dosages often stay under 5 ppm. In beverages or confections aiming for subtler brown or roasted flavors, the dial cranks down further, often landing near or below 1 ppm.
Some flavorists argue the safer bet comes from starting even below that and inching up until the blend clicks. I’ve pipetted standards for hours just to help a chief flavorist tweak a formula, moving one-tenth of a microliter at a time in a pilot blend. Most household cooks never think about ratios this tiny, but for these powerful ingredients, less really is more.
High potency brings big responsibility. 2-Methylthio pyrazine can make or break a food’s flavor profile, especially since people’s noses pick up sulfur and roasted aromas at vanishingly small levels. Overshooting creates bitterness and a musty edge, or drowns out other important notes. Snack companies have faced product returns and blown launch deadlines due to these small errors. That hits the bottom line and can hurt the brand reputation.
Fact: Regulatory bodies like the FDA and EFSA label this compound GRAS (Generally Recognized as Safe) at standard use levels, but safety studies reinforce low exposure for consumers as a sensible target. No one insists on these levels just for paperwork reasons; consumer panels pick up “off” notes very quickly, and sales numbers show the fallout when a flavor profile shifts away from what people expect.
Labs today use microgram-scale balances and autosamplers to avoid guesswork, and flavor software helps track cumulative concentrations when multiple pyrazines appear in one recipe. Some companies lean on natural extraction methods to combine several related notes, blending them with synthetic additions to build complexity without needing a heavy hand. Others stress routine sensory panels—actually tasting samples with staff trained to spot too much or too little pyrazine—getting human judgment involved before things hit the shelves.
From my experience, nothing beats a small-scale pilot run and plenty of patience—a few tweaks, a little time for the blend to mature, and an honest taste before pushing “go.” The tools matter, but a careful, low-dose mindset saves the day.
Most people probably haven’t heard of 2-Methylthio Pyrazine unless their world spins around flavors, fragrances, or chemicals in a lab. This stuff may not turn heads at the grocery store, but it means business behind the scenes—usually giving foods those roasted, nutty or earthy smells that make us hungry. Despite sounding niche, this compound deserves respect in storage like any chemical with a bit of character. I’ve spent enough time near busy lab benches and smelly storerooms to know how easy it is to mess up with aromatic compounds.
One thing just about anyone working with 2-Methylthio Pyrazine can tell you: its intense aroma survives just about anything. Spill a tiny drop and you’ll be smelling it for days. This isn’t just annoying. Leaky storage means wasted product and a room full of folks searching for the source of “that weird smell.”
Temperature acts as the most basic line of defense. These types of pyrazines don’t handle heat well. Some suppliers suggest room temperature is fine, but from my experience, “room temperature” can swing pretty wildly. Fire up the heating in winter and you could easily tip toward 30°C, which encourages decomposition or reactions with air. Keep your container cool, below 25°C if possible, so the compound stays fresh and doesn’t get aggressive or weird-smelling. I tend to stash aroma chemicals like this on lower shelves, far from radiators, and never near strong sunlight.
If you pop open a jar of 2-Methylthio Pyrazine a dozen times a week, you’re practically inviting air in to start messing around. Over time, oxygen can take some of the punch out of your compound or start chemical changes that muddy the aroma. It’s much safer using containers with snug, secure lids.
People sometimes overlook humidity. Even if it isn’t hydroscopic (it doesn’t suck up water from the air aggressively), 2-Methylthio Pyrazine lasts longer where it’s dry. Moisture brings trouble by attracting unwanted chemistry, making things sticky, or even growing little bits of mold if another contamination gets in. Throw in a silica gel pack if you want that extra bit of dryness.
Plastic can play tricks on you—some of it soaks up smells and stains easily, which means you lose product or cross-contaminate batches. I trust glass, especially with tight, chemical-resistant caps. Glass doesn’t react or hang on to odors between uses, and one accident taught me that even food-grade plastics can get ghost aromas that taint the next thing you store. Stainless steel, though tough, risks subtle reactions if left for years, so glass wins for me every time.
Organization helps. I once lost a whole batch because someone stuck the jar right next to a heat-generating fridge. Label everything with storage dates and temperatures. Rotate your stock so old stuff gets used up first. If the warehouse gets humid in rainy seasons, set up a dehumidifier and save yourself the trouble of ruined stock.
If there’s ever a doubt about safety or quality, regular checks go a long way. Smell your chemical, inspect for any weird layering or lumps, and keep a proper log—not just for inspectors, but for your own sanity when retracing storage mistakes. Toss anything that’s off instead of risking a ruined recipe or a spoiled batch.
Good storage raises shelf life and keeps everyone in the building happier. With a little care, 2-Methylthio Pyrazine behaves well, saves costs, and does its aromatic job without taking over your workspace. It’s a lesson in respect—maybe for chemistry, but mostly for practical habits anyone can pick up with a bit of practice.
Walk through a bakery or open a bag of roasted nuts, and you’ll catch whiffs of scents that feel like home. Some ingredients behind those flavors come with names that barely fit on a label. 2-Methylthio pyrazine is one of them. This molecule packs a punch with its roasty, earthy, almost nutty character. People working in food flavoring or fragrance often ask—does it come from nature, or do we make it in a lab?
2-Methylthio pyrazine belongs to a group called pyrazines, known for contributing to flavors people recognize in coffee, popcorn, and chocolate. These molecules show up in small amounts when heat transforms sugars and proteins—think browning bread or grilling meat. During these reactions, nature stitches together all sorts of pyrazines, some with a faint odor, others that could fill a kitchen with just a whiff.
I’ve spent years reading up on ingredients and talking with food scientists who balance what’s real from what’s made. 2-Methylthio pyrazine does show up naturally. You’ll find tiny traces in roasted nuts, cooked meat, and coffee beans. But here’s the catch—the amounts in nature are minuscule. It takes thousands of pounds of roasted peanuts to harvest just a smidge of this compound, nowhere near enough for industry-wide flavor use.
So, the food world often turns to smart chemistry. Through a controlled process—mainly using heat and precursors like sugars and amino acids—chemists can put together 2-methylthio pyrazine with precision. The compound built this way matches what’s in roasted foods: same structure, same smell. Most of what ends up in snacks, seasonings, or even perfumes starts in a lab, not a field.
Many shoppers get nervous seeing chemical names, believing natural equals safe and synthetic equals risky. The truth isn’t so tidy. Just because something arrives in a test tube doesn’t mean it’s bad. For instance, synthetic vitamin C keeps people healthy—nobody expects every dose to come from oranges. The issue with flavor molecules like 2-methylthio pyrazine is transparency and trust. Companies need to be up-front; people have a right to know what they’re eating.
Regulators in Europe and the US recognize synthetic and nature-identical forms as safe for use in food, provided they meet purity standards. The science backs this up; human bodies break down the synthetic version just like the natural one. But labeling rules lag behind consumer curiosity. Many flavor ingredients get tucked under phrases like “natural flavoring” or “artificial flavor.” If shoppers want more clarity, laws should require ingredient lists that spell out these details. Food brands gain trust by demystifying these decisions rather than hiding behind technical terms.
Some chefs and craft producers still lean on traditional roasting and extraction to capture flavor the old-fashioned way. This method respects the complexity and artistry that goes into food. Large-scale manufacturers, on the other hand, rely on synthetics for consistency, cost, and availability. There’s space for both: tradition for small batches and science for feeding millions.
If people ask whether 2-methylthio pyrazine is natural or synthetic, the answer depends on the source—and the scale. In most products, it starts in a lab, designed to mimic what nature makes, right down to the molecule. Being informed, asking questions, and pushing for clear labels can help everyone make choices that fit their values and tastes.
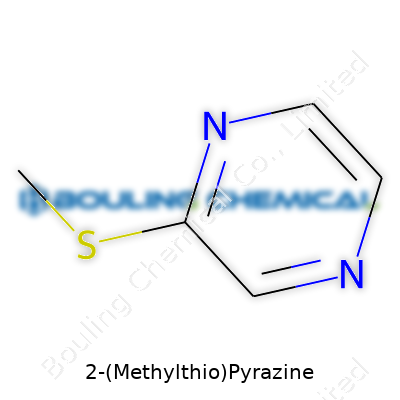
| Names | |
| Preferred IUPAC name | 2-(Methylsulfanyl)pyrazine |
| Other names |
2-Methylsulfanylpyrazine 2-(Methylthio)pyrazine Pyrazine, 2-(methylthio)- |
| Pronunciation | /tuː ˈmɛθəlˌθaɪoʊ paɪˈreɪzin/ |
| Identifiers | |
| CAS Number | [28525-90-5] |
| 3D model (JSmol) | `3Dmol.js('#app', {width: 400, height: 400}, function(viewer) {viewer.addModel('CC1=CN=NC=C1SC', 'smi');viewer.setStyle({}, {stick:{}});viewer.zoomTo();viewer.render();});` |
| Beilstein Reference | 1203886 |
| ChEBI | CHEBI:134435 |
| ChEMBL | CHEMBL3177976 |
| ChemSpider | 28759 |
| DrugBank | DB03217 |
| ECHA InfoCard | ECHA InfoCard: 100_013_995 |
| EC Number | 209-104-9 |
| Gmelin Reference | 852595 |
| KEGG | C16306 |
| MeSH | D017929 |
| PubChem CID | 13553 |
| RTECS number | UJ8220000 |
| UNII | J8V44R9Y6P |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | DTXSID4039243 |
| Properties | |
| Chemical formula | C5H6N2S |
| Molar mass | 154.21 g/mol |
| Appearance | Yellow to brown liquid |
| Odor | Nutty, Roasted, Popcorn, Coffee |
| Density | 1.107 g/mL at 25 °C |
| Solubility in water | Insoluble in water |
| log P | 1.08 |
| Vapor pressure | 0.0415 mmHg at 25 °C |
| Acidity (pKa) | pKa = 2.20 |
| Basicity (pKb) | 2.10 |
| Magnetic susceptibility (χ) | -61.6·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.573 |
| Dipole moment | 1.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4474.0 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: H302-H319-H335 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 86°C |
| Lethal dose or concentration | LD50 (oral, rat): 730 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 2-Methylthio Pyrazine: "930 mg/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.01 |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
2-Methylpyrazine 2-Ethylthio pyrazine 2-Methylthio-3-ethylpyrazine 2-Acetylthio pyrazine 2-Isopropylthio pyrazine |