Back in the early twentieth century, chemists scoured the vast families of nitrogen heterocycles, hunting for members that could push pharmaceutical and agricultural chemistry forward. 2-Methylpyrrolidine showed up on the bench as a rather overlooked ring, overshadowed by its cousin, pyrrolidine. Once synthetic routes to pyrrolidines evolved past the old pyrolysis and alkali reduction of amides, researchers began tinkering with substituent patterns. This modest methyl group, attached at the second ring position, opened doors to whole new categories of synthons and end products. From personal experience inside an R&D lab, conversations often turned to how every tiny tweak in a molecule’s skeleton can shift biological activity or change how it blends into a formulation, and the rise of 2-methylpyrrolidine offers a textbook example.
2-Methylpyrrolidine steps up as a fundamental building block. Drug companies lean on this molecule as a chiral auxiliary, helping steer reactions toward the right-handed or left-handed product. Agrochemical companies, always looking for new plant growth regulators or protective agents, have steadily increased orders for this tiny amine ring. Rather than simply list it as another organic solvent or intermediate, some teams rely on it for physical properties—low boiling point, manageable polarity, basicity—for purification steps. The product rarely makes headlines, but its fingerprints rest on patents covering everything from anti-infectives to industrial catalysts.
2-Methylpyrrolidine presents itself as a clear, colorless liquid with a distinct, sharp odor—if you’ve spent any time in a synthesis lab, it’s a scent not easy to forget. The molecular formula C5H11N packs a modest weight of 85.15 g/mol. This amine shows moderate water solubility and mixes easily with typical organic solvents such as ether or benzene. Its boiling point—90–92°C—lets it distill without drama on a small scale, and its vapors linger in open air. Anyone handling this material quickly notices its basicity, which partners well with alkylation, acylation, and reductive amination reactions. Hydrogen bonding still arises, but not nearly as strong as in pyrrolidone relatives. Chemically, the methyl group stabilizes the nitrogen’s lone pair just enough to keep unwanted side reactions at bay in multi-step syntheses.
On most reagent shelves, bottles of 2-methylpyrrolidine carry labels with CAS number 120-94-5, batch purity above 98%, and listed moisture content below 0.5%. You'll spot hazard markings plain as day—flammable liquid, corrosive to skin and eyes. What always catches attention is the need for nitrogen-purged storage to block oxidation, and a tightly closed cap thanks to its volatility. Large-scale purchases usually bring batch analysis sheets covering GC-MS traces for byproducts and isomer content, as even a fraction of pyrrolidine or higher methylated species can skew sensitive downstream chemistry.
In practice, most 2-methylpyrrolidine comes from catalytic hydrogenation of 2-methylpyrrole. Chemists crank up the heat and pressure in the presence of a Raney Nickel or platinum catalyst, guiding hydrogen atoms into the ring to saturate double bonds. From my time scaling a reaction in the pilot plant, I remember how tricky it got if the starting pyrrole carried impurities—trace aldehydes or poorly washed acids guaranteed extra headaches at the distillation stage. Alternate methods, like reductive amination of 2-pyrrolidone with methylamine, pop up in patents but never really crossed over to bulk manufacture.
As a secondary amine, 2-methylpyrrolidine responds to classic amine chemistry—it picks up acyl or alkyl groups cleanly, works as a nucleophile in SN2 substitutions, and stands up fairly well to mild oxidants if conditions stay controlled. Methylation on the nitrogen offers a pathway to tertiary amines, which crop up all through central nervous system drug design. The ring itself occasionally faces attack from strong electrophiles but, more often, serves as a platform for chiral catalysis. In asymmetric hydrogenations, enantiopure derivatives drive high selectivity, a detail synthetic chemists sweat over to avoid expensive chiral separations later.
Depending on the context, you might spot it sold under different vendor names: 2-Methyl-tetrahydropyrrole, N-Methylpyrrolidine, Tetramethylene methylamine. The pharmaceutical world sometimes tags it as “MePyrr” in process development notes. In chemical catalogs, searching for “2-Methylpyrrolidine, 97%” usually brings up the right bottle, but I’ve seen teams cross wires by mistaking it for its N-methylated or 3-methyl isomers—a safety and reactivity headache not worth repeating.
Safety officers remind everyone that 2-methylpyrrolidine isn’t exactly benign. Its volatility means splash goggles, gloves, and lab coats show up as basic requirements every time someone cracks open a bottle. Skin and respiratory irritation get reported if fume hoods aren’t running strong. Fire departments stress the need for grounded containers and spark-resistant fans in storage areas, since this amine’s flash point sits at just 13°C. Accidental spills feel more dramatic than they look—vapors rise fast, so it pays to work below chest height with proper ventilation. Disposal gets stricter by the year. Waste handlers ask for organic amines to go into hazardous waste streams, never down the drain or trash.
Pharmaceuticals absorb most of the annual production, as 2-methylpyrrolidine helps build scaffolds for drugs against central nervous system disorders and cancer. Its ring slipstreams into synthesis steps where selectivity matters—making chiral intermediates for API synthesis. Agrochemicals grab their share, especially for herbicide and fungicide formulation studies where novel side groups help dodge resistance. Academic labs still pick it up for mechanistic studies on ring strain or nitrogen basicity, and specialty polymers sometimes rely on it for end-group control. My own time in industry saw this molecule pop up when other amines lost effectiveness or demanded extra safety controls, so it rarely lands as a “first choice” but delivers where it counts.
Organic chemists dig into 2-methylpyrrolidine when searching for novel asymmetric reactions. Grant proposals point out the ability of this amine to steer catalyst selectivity or add a twist to a synthetic toolbox. Research focuses on greener synthetic pathways, hoping to drop hazardous reducing agents and cut down waste streams. Papers report new roles for the ring in metal-ligand designs, especially for nickel and palladium complexes hunting for better turnover numbers in fine-chemical manufacture. University teams recently examined how the small methyl group shunts electron density in predictable ways, shaping the molecule’s reactivity profile. Already, more undergraduate research projects tackle the stereochemical impact of this methyl substitution on pharmacologically active analogs.
Toxicologists warn of the dangers of unchecked exposure. Rat studies show moderate acute toxicity—oral LD50 often falls near 300–600 mg/kg. Skin absorption remains a real risk; repeated handling without gloves brings chronic effects such as dermatitis and dizziness. A handful of studies explore its metabolism in mammals—cytochrome P450 enzymes break it into carboxylated metabolites, which leave the body via urine, but these pathways risk creating more reactive intermediates along the way. Regulatory reviews follow the usual playbook: industrial exposures must fall under strict OSHA and REACH thresholds, and environmental emissions get tracked closely since amines can disrupt aquatic life at surprisingly low concentrations.
Looking ahead, chemists remain optimistic about dialing in new uses for 2-methylpyrrolidine, especially as companies chase chiral purity in both drug and agrochemical synthesis. Efforts center around greener and more efficient extraction and hydrogenation processes—solventless setups and recyclable catalysts headline recent symposiums. Ongoing work to tame its volatility and flammability through safer packaging and microdosing tech could open new application routes. Companies with deep roots in fine chemicals see promise in pairing it with automated flow chemistry, seeking tighter quality targets while cutting worker exposure. As regulations stiffen and sustainability counts more every year, this classic amine still carves out its role—one careful experiment and one tightly capped bottle at a time.
Ask around in any lab that works with 2-Methylpyrrolidine and you quickly learn purity almost always comes out at 98% or above. Some suppliers brag about 99% levels. Pharmaceutical companies and chemical researchers alike keep their expectations this high because lower grades can wreck the consistency of reactions and cost real money in lost yields or botched batches.
My own experience with 2-Methylpyrrolidine taught me that even a single muddy impurity can shift reaction profiles or toss off a stench that lingers for days. This isn’t just textbook paranoia—these subtle differences change things on a molecular level. I spent a few years working on heterocyclic synthesis. During that time, anything under 98% led to unwanted byproducts that took hours to separate. That’s labor, solvents, time, and nerves lost.
In organic synthesis, especially where chiral centers get involved, a poison impurity means much more than a smudge on your purity certificate. Impure 2-Methylpyrrolidine can poison a catalyst, quench a reaction midstream, or slip into the final product when you just want one clean signal in your NMR.
Companies like Sigma-Aldrich or TCI issue a Certificate of Analysis with every batch. Some labs run their own tests, sometimes just to double-check. Liquid chromatography, NMR, even gas chromatography all tell the story if something isn’t right. Nobody wants to gamble thousands of research dollars, so buying the highest grade puts those nerves to rest.
Lower purity means more work. Imagine this: you’re wrapping up a multi-step synthesis, and the impurity from your starting 2-Methylpyrrolidine suddenly shows up after isolation. You spend all Thursday and Friday trying to purify your product, scrapping what should have been a clean reaction. Labs everywhere see these extra cleanup steps pile up, driving up costs.
Scalability becomes much harder, too. A pharmaceutical pilot plant once had to pull back an entire line when the input chemical—advertised as “pure”—crept in at 94%. Management blamed the lab staff, but a quick check showed the supplier had switched up their production process. Trace junk in the solvent phase climbed up the column and ruined everything downstream.
Researchers have to work closely with suppliers. I started requesting batch samples to run my own checks before committing to a large order. Direct communication with sales reps sometimes flags up a bad batch before it goes out. Some labs pool purchases together, pushing for guaranteed higher specs at a lower price.
It helps to track every problem that crops up. Whenever a reaction stalls or purification gets messy, point to the batch. Over time, patterns emerge. Sometimes, convincing your purchasing department takes a stack of ruined reactions and column runs, but logging each instance pays off.
Most suppliers now offer 98% as standard, with higher grades costing a bit more. Every chemist I know would rather shell out extra upfront than spend days sorting out avoidable impurities. So while sales brochures often shout about highest grades, it’s still smart to test what you get—trust, but verify, as the saying goes. The cost of sloppier material always catches up, whether it’s in missed deadlines, lost material, or just pure frustration.
Ask anyone in a chemistry lab what they fear most—chances are, it’s not the fancy, break-the-bank equipment. It’s the headache of spoiled chemicals. I’ve known 2-Methylpyrrolidine to develop ugly colors and odd smells when neglected, not just becoming useless but also leading to real hazards. This one’s no pantry sugar; it comes with volatility and reactivity that demand respect.
From direct experience, leaving 2-Methylpyrrolidine on an office shelf or anywhere near sources of heat sets you up for trouble. Heat encourages evaporation and makes those flammable vapors more aggressive. Refrigerators reserved for chemicals, set just above freezing—think 2°C to 8°C—work wonders for reducing loss and risk. In everyday labs, it’s common practice to avoid keeping this stuff within striking distance of any hot equipment, including radiators and windows flooded with sun.
Clear glass bottles left under fluorescent lab lights never last long. Light speeds up decomposition, which not many first-year students realize until a supervisor frowns at a ruined sample. Amber bottles and opaque containers help block out most unwanted rays. I’ve always favored storing sensitive organics in tightly closed containers, squeezing the air out when possible—less oxygen slows down unwanted reactions. Sealing containers also helps fight evaporation, a serious issue with volatile organic liquids.
Water creeps in wherever it can, and with 2-Methylpyrrolidine, even small amounts spell disaster. From rusted caps to reacted product, keeping everything bone-dry preserves both the purity and safety of the material. I keep desiccators and packets of silica gel around, especially in shared spaces where someone forgets to screw a lid back on. Leaks don’t just degrade chemicals; they turn storage cabinets into clean-up zones.
Here’s a hard rule from my own scuffed notebook: never store flammables like 2-Methylpyrrolidine with oxidizers. It sounds obvious until you see someone line up peroxide bottles with amines on a single shelf. Safety cabinets for flammable chemicals, fitted with self-closing doors and proper ventilation, protect lives as much as property. Sprinklers and fire extinguishers in arm’s reach—checked, not just installed—can make all the difference between a close call and a disaster.
It’s tempting to grab the marker and scrawl a half-legible abbreviation on a bottle. But with clear labels, including the date received and opened, you catch tired chemicals before they turn against you. A good storage log, checked during regular audits, avoids confusion and keeps dangerous surprises in check. Older stock, moved to the front of a storage cabinet, gets used first, and nothing accidental gets pushed to the back to stew.
Good storage conditions for 2-Methylpyrrolidine mean more than just following a list. From temperature control to moisture blocks and safer layouts, the right habits combine personal vigilance and shared responsibility. Practical steps—like using the right fridge, sealing containers tightly, and running those regular checks—stop problems before they start. That’s not procedure for procedure’s sake. In my view, it’s the only way to protect not just the chemicals but everyone who handles them.
Step into a research lab or a chemical plant, and you’ll probably catch a whiff of chemicals you’d never hear about on the street. 2-Methylpyrrolidine is one of those names you don’t find in regular conversations, but it quietly plays a big supporting role behind plenty of things people use every day. Its energy comes from that little nitrogen ring structure—something chemists get excited about for good reasons.
You take a painkiller, a blood pressure pill, or maybe an antidepressant. Most people never ask what goes into making those tiny tablets so reliable. Manufacturing them calls for clever building blocks, and 2-Methylpyrrolidine stands out as one of those helpful sidekicks in drug discovery. Its structure makes it handy when drug chemists want to tweak molecules, making drugs more effective or less likely to break down too soon. That five-membered ring can nudge a drug’s activity in just the right direction. In some cases, chemists use it directly to whip up HIV medications or experimental cancer treatments. Without chemicals like this, designing better medicine would take a lot longer—and cost a lot more.
Out in the field, farmers face bugs, weeds, and other threats to their harvest. Crops need a boost to fight off these problems and produce more food. 2-Methylpyrrolidine helps here too. It serves as a stepping stone to pesticides and herbicides, where scientists fine-tune the effects so crops thrive and pests don’t. I’ve watched agronomists experiment with new agrochemicals season after season, and almost every time, there’s some nitrogen-based compound quietly making the recipe work. It keeps production up without leaving a heavy footprint on the ground, which matters as much for the soil as for our plates.
Take a look around your home: paints on the walls, coatings on kitchen tools, or adhesives holding together the things you didn’t even know needed glue. Chemicals like 2-Methylpyrrolidine step into these areas, too. Certain polymers and advanced plastics begin with this compound, which brings in flexibility, resistance, or special surface properties. A manufacturer might reach for it to create something tough enough for your new smartphone’s casing or clear enough for optical fibers tucked under city streets.
Lab chemists look for molecules that can speed up reactions or help them build complex molecules. 2-Methylpyrrolidine often finds its spot on a crowded shelf, ready to show what it can do as a reagent or a catalyst—for example, helping researchers develop new antibiotics or more efficient solar panels. Experienced scientists like to have chemicals with nimble reactivity around because they jump-start experiments that would otherwise limp along.
The way we design medicine, feed our communities, and build the next generation of materials all depend on small players like 2-Methylpyrrolidine. Production needs tight regulation, of course, since chemical safety can’t slip through the cracks. Producers must keep emissions low and ensure workers have the right training and safety gear. For those looking to the future, green chemistry matters. Investing in ways to make and use chemicals with less waste and fewer risks means 2-Methylpyrrolidine may keep helping in more ways than expected.
People in labs and industries handle all sorts of chemicals every day. If you’ve ever spent time mixing reagents or cleaning glassware, you start taking those colored warning symbols seriously. A safety data sheet, or SDS, spells out everything someone needs to know to work safely with a substance. This includes what the chemical does to your body, how you should store it, cleanup rules, and emergency steps if someone spills or swallows it. I’ve seen accidents happen when folks assumed they’d “figure it out” and skipped the research. Chemicals like 2-Methylpyrrolidine don’t forgive carelessness.
2-Methylpyrrolidine isn’t as popular as something like acetone or ethanol but pops up in organic synthesis labs and research settings. It has the kind of sharp, amine-like odor that sticks in your memory the first time you open a bottle. The SDS gives warnings about flammability, possible irritation, and steps for proper disposal. Digging through university databases and supplier resources shows that decent SDS documents for this compound exist from companies like Sigma-Aldrich and Fisher Scientific. Even in 2024, you sometimes need to dig a few clicks deeper, but if you search for “2-Methylpyrrolidine SDS” plus the name of a chemical supplier or state regulatory agency, you’ll come up with several detailed sheets.
During a summer internship, our group worked with methylated amines. Small mistakes turned into big headaches. One time, a sealed vial containing residual vapors pressurized and the snap startled everyone in earshot. It reminded us that pressure buildup and volatility aren’t just lab textbook warnings. Even a modest spill stinks up a room and triggers eye and throat irritation. The SDS for 2-Methylpyrrolidine would list these risks clearly: use fume hoods, gloves, goggles, lab coats; always check and double-check for leaks or improper seals.
Most researchers learn quickly that shortcutting safety wastes time and money later. Broken glass, contaminated surfaces, and exposure to toxic vapors turn into lost experiments and sometimes medical bills. The SDS isn’t just for regulatory paperwork. It’s the best shot anyone’s got at stopping disaster before it starts, especially with compounds that don’t give much warning before causing harm.
Smaller labs sometimes miss out on the most up-to-date information, especially with rare compounds like 2-Methylpyrrolidine. There’s a tendency to fall back on supplier labels or older binders in a cabinet that nobody’s touched in years. Older sheets might still mention outdated PPE guidelines or miss recent toxicology research. People onsite deserve better. SDS updates should get circulated by email or app notification, not just tape on a wall. Supervisors can help by making sure their teams stop and check the latest SDS before opening any fresh container.
Anyone handling chemicals, from undergrad lab techs right up to PhD researchers, should treat the SDS as essential reading. Digital systems that let you scan a barcode and see the up-to-date sheet help close the information gaps. Safety officers and professors can encourage short reviews or even pop quizzes so folks keep learning instead of treating the SDS like busywork. It’s not about feeding compliance checklists—it puts the knowledge right in people’s hands before an accident can ruin a project or a person’s health. Efforts to improve chemical safety should focus on fast, clear, and ready access to these sheets for every single chemical, every single day.
There’s an old saying in production: missing a shipment can be just as bad as shipping the wrong product. For buyers and planners dealing with 2-Methylpyrrolidine, timing matters. I used to work in supply chain for a small specialty chemicals distributor. Every time we had a spike in demand or a surprise audit, getting our hands on key chemicals on time kept the lab running, and our customers happy. Waiting two weeks instead of four can keep a project alive. Long lead times, on the other hand, can push deadlines, run up costs, or just create headaches nobody needs.
For 2-Methylpyrrolidine, most suppliers aim for a lead time around 2–4 weeks if the product is on their regular rotation and no customs issues block the way. Some bigger distributors stock common package sizes, which means overnight or a few days is possible, but special sizes or purity grades can push that lead time out. During COVID or port slowdowns, lead times drifted closer to 6–8 weeks. Emergencies like lab accidents only underline how delays in delivery can ripple out and cause real messes.
Any lab tech who has sliced open an awkwardly sized drum of solvent knows packaging affects workflow as much as content. 2-Methylpyrrolidine doesn’t just show up one way—it usually gets delivered in smaller glass or HDPE bottles (500ml, 1L, 2.5L), mid-size jerry cans (5L, 10L, sometimes 20L), or metal drums for larger orders. Most of the time, these are sealed tightly with tamper-evident caps, usually packed in strong outer boxes or crates to stop leaks during transit.
In my lab days, glass bottles worked best for careful bench work and small batch experiments. Our production-scale synthesis ran smoother with 10L or 20L HDPE drums, since HDPE holds up well against most solvents and weighs less for carrying down a hallway with gloves on. The choice depends not just on how much you need, but also on how easy it is to pour, store, and handle. Using the right package keeps you safe—since 2-Methylpyrrolidine can irritate and evaporates quickly—and stops waste.
Package size and lead time are tied together. If you ask for a rare drum size or a custom liner, expect to wait. Suppliers streamline operations by sticking to a few standard options—easier for storage, faster to ship. Ordering standard sizes bumps you to the front of the queue. I’ve seen buyers land themselves in a supply crunch by chasing custom packaging, only to find out it doubled their wait and added fees they didn’t budget for.
The other problem is minimum order quantities. If you only need a liter or two and the supplier sets a higher minimum, costs climb fast. Small volume buyers—in universities, for example—often share shipments to stretch budgets and keep waste down. Bigger companies stick to bulk drums for cost savings, but face more risk if lots go off spec.
Direct communication with suppliers helps avoid surprises. It’s better to check lead times before setting project timelines. Ask what’s in stock. Request packaging details: Are caps leak-proof? Any hazard labels included? Find out about backup options in case regular lines run dry. Some labs even keep emergency stock for essential chemicals like 2-Methylpyrrolidine. Insurance against delays beats scrambling at the last minute.
The heart of supply chain for chemicals isn’t just about moving drums and bottles. People need to plan cautiously, weigh risks, and treat packaging as part of safety, not just delivery. For 2-Methylpyrrolidine, the difference between a smooth order and a costly mistake often comes down to responsive suppliers, realistic timelines, and good old-fashioned planning ahead.
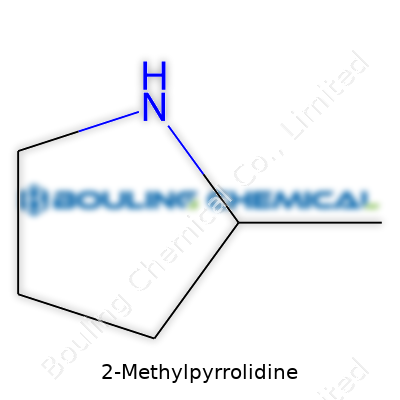
| Names | |
| Preferred IUPAC name | 2-Methylpyrrolidine |
| Other names |
2-Methyl-1-pyrrolidine 2-Methylpyrrolidine 2-Methyl-pyrrolidine |
| Pronunciation | /tuː-ˈmɛθ.ɪl.pɪˈrɒl.ɪˌdiːn/ |
| Identifiers | |
| CAS Number | 96-43-5 |
| Beilstein Reference | 1209242 |
| ChEBI | CHEBI:16024 |
| ChEMBL | CHEMBL147094 |
| ChemSpider | 11764 |
| DrugBank | DB04109 |
| ECHA InfoCard | echa.europa.eu/infocard/100_007_335 |
| EC Number | 872-90-2 |
| Gmelin Reference | 7876 |
| KEGG | C05907 |
| MeSH | D051520 |
| PubChem CID | 12363 |
| RTECS number | SY8225000 |
| UNII | 82FI9T4C1D |
| UN number | UN2555 |
| Properties | |
| Chemical formula | C5H11N |
| Molar mass | 85.15 g/mol |
| Appearance | Colorless liquid |
| Odor | amine-like |
| Density | 0.867 g/mL at 25 °C |
| Solubility in water | miscible |
| log P | 0.16 |
| Vapor pressure | 3.8 kPa (at 20 °C) |
| Acidity (pKa) | 11.3 |
| Basicity (pKb) | pKb = 2.93 |
| Magnetic susceptibility (χ) | -13.10 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.427 |
| Viscosity | 0.773 cP (20°C) |
| Dipole moment | 2.11 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | S⦵298 = 298.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −83.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3888.7 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H225, H302, H314 |
| Precautionary statements | Precautionary statements of 2-Methylpyrrolidine: "P210, P233, P260, P271, P280, P303+P361+P353, P304+P340, P312, P403+P235, P501 |
| NFPA 704 (fire diamond) | 2-Methylpyrrolidine NFPA 704: 2-3-0 |
| Flash point | -7 °C (19 °F; 266 K) |
| Autoignition temperature | 460 °C |
| Explosive limits | 1.6–10.4% |
| Lethal dose or concentration | LD50 oral rat 193 mg/kg |
| LD50 (median dose) | LD50 (median dose) for 2-Methylpyrrolidine: "Mouse oral LD50 = 200 mg/kg |
| PEL (Permissible) | No PEL established. |
| REL (Recommended) | 10 mL |
| IDLH (Immediate danger) | IDLH: 100 ppm |
| Related compounds | |
| Related compounds |
Pyrrolidine N-Methylpyrrolidine 3-Methylpyrrolidine 2,5-Dimethylpyrrolidine Pyrroline |