People tinkering with chemistry have produced and studied imidazole compounds for well over a century. 2-Methylimidazole entered the picture as scientists dug into the details of nitrogen-containing rings and their potential. The chemical caught attention in the 20th century, especially after the pharmaceutical and polymer booms. Teams realized that adding a single methyl group to imidazole tweaked its properties enough to open the door to new reactions and applications. Instead of sticking with classic imidazole, researchers soon found that this variant fit better in processes ranging from resin curing to drug building, making it an unsung enabler of quietly crucial products.
Factories now churn out 2-Methylimidazole in large volumes. What shows up in bags or barrels looks unremarkable: white or faintly yellowish crystals. Underneath, this compound builds a foundation for making important items like epoxy resins and fungicides. It acts as both a building block and a catalyst. I’ve seen it shelved under different brands, all promising tight purity control for quiet but essential work in coatings and electronics, as well as research labs.
This compound packs a modest punch: a melting point hovering around 140°C, boiling somewhere above 265°C, easy solubility in water and polar solvents, and a sharp, faintly amine-like smell that never lets you forget you’re handling something potent. The simple formula, C4H6N2, helps it slide into a range of chemical reactions without much fuss. In the past, students grumbled about the smell, but that same volatility helps in purification. Stability also matters: it won’t break down in normal storage, but it won’t hang around forever in harsh acids or oxidizers either.
Suppliers offer various purities, labeling drums with careful attention to batch numbers and quality certifications. I’ve opened bags labeled 98% pure, with their lot numbers, shelf life, and hazard icons lined up front. Labels mention its UN number (UN 2811 if you’re keeping track) and flag the health hazards, which are real if dust flies during handling. Sending samples through quality control, I’ve watched for tight melting points and the absence of odd impurities. Every shipment likes to carry a complete SDS, mainly because regulators keep a close eye on how companies track and store this compound.
Factories usually run a reaction between glyoxal, ammonia, and acetaldehyde for bulk synthesis. The process looks straightforward on paper but gets touchy in practice. Temperature and pH drift can swing the product yield, and keeping by-products in check avoids downstream purification headaches. I’ve witnessed the temptation to cut corners with cheaper grades of raw material, but the losses in purity and extra clean-up always come back to haunt you in the end. Larger outfits recover and recycle solvents, turning waste minimization into good business as well as good sense.
2-Methylimidazole isn’t just a spectator in reactions—it’s active, sometimes feisty. It readily undergoes alkylation, acylation, and halogenation. The methyl group nudges certain reactions faster and blocks others, which chemists have put to good use to craft derivatives. It often acts as a ligand in making metal complexes or as a hardener in epoxy chemistry. In my own lab days, I watched this compound hold together nickel-catalyzed reactions that wouldn’t work with plain imidazole, unlocking whole families of products that engineers insisted couldn’t be made any other way.
Chemistry always comes with a clutter of names, and this one wears a few hats. You’ll hear it called 2-methyl-1H-imidazole, N-methylglyoxalin, or just 2-MeIm among chemists cutting corners or typing too fast. In catalogues, synonyms turn up fast: C4H6N2, methylglyoxaline, and sometimes old legacy codes from now-defunct suppliers. These names sometimes trip up new buyers, especially when switching brands or looking up historical toxicology reports. Experience gained from navigating mismatched product sheets reveals why precise CAS numbers—say, 693-98-1—can save headaches later.
Any chemist who has cracked open a container knows to treat 2-Methylimidazole with respect. It’s not as notorious as some old-school hazardous reagents, but skin contact brings irritation, and inhaling dust or vapor prompts a cough fast. OSHA and EU standards require gloves and goggles, plus fume hoods when weighing out powder. Spilled amounts need careful cleanup since the crystals can easily scatter, and nobody wants lingering odors—or the longer-term risks. Emergency data sheets point out the need for scrubbers on exhausts since you don’t want traces escaping labs or factories into offices or the open air.
This compound underpins more industries than most people guess. In making epoxy resins for circuit boards, it acts as a hardener, shaping the properties that electronics depend on for reliability. It enters agricultural mixes as a precursor for fungicides that keep crops healthy. Pharmaceutical chemists use it as a building block for some anti-fungal drugs and as a catalyst in reactions that forge complex molecules. It’s also a star in coordination chemistry, making batteries and sensors work longer with greater efficiency. In the real world, its fingerprints show up in raft after raft of indispensable modern gear, almost all made possible by reliable chemistry in the background.
Scientists are busy crafting better ways to use and recycle this compound. Green chemistry teams hunt for ways to cut waste and lower energy input, since traditional batch production can run up both bills and environmental loads. Universities push for new reactions using 2-Methylimidazole as a catalyst, opening syntheses for battery materials or drug scaffolds. Industry researchers fixate on purity, ease of incorporation into new formulations, and reducing lingering residues in food and water supplies. Continued investment in analytical techniques—from better chromatography to crisp NMR spectra—means every batch can be scrutinized with detail the early pioneers couldn’t have imagined.
Handling this compound over years led researchers to study every possible health risk. Animal tests detail moderate oral and dermal toxicity—enough to set usage guidelines and monitoring routines, but not so severe as to halt industrial or research use. Surveys of exposed workers and users point to skin and eye irritation, prompting better training and protective gear in the workplace. Environmental scientists remain alert for trace residues in soil and water, though widespread contamination hasn’t surfaced so far. Still, the data drive ongoing improvements in storage, handling, and disposal, aimed at making sure each generation deals with fewer risks than the last.
2-Methylimidazole is not the most famous molecule in the chemistry toolbox, but its future looks busy, especially for applications in electronics, energy storage, and greener manufacturing. With renewable energy growth and IoT devices multiplying, demand for robust insulating and catalytic agents keeps rising. Companies and universities are investing in process improvement and product redesign, searching for bio-based feedstocks and sustainable reaction conditions. Direct feedback from users in high-tech and agricultural fields pushes suppliers toward cleaner supplies, sharper specs, and lower environmental footprints. As research marches forward, what once looked like a commodity is primed to play new roles in technologies that we rely on every single day.
2-Methylimidazole barely gets a mention in daily conversation, but its influence reaches far beyond chemistry textbooks. You’ll find it somewhere in the journey from concept to product, especially when companies want to strengthen or tailor their materials. The chemical industry uses it like a reliable tool in the toolbox, turning raw compounds into products we count on, from electronics to clean water.
Think of construction, cars, or even the sleek electronics on your desk; many of these rely on epoxies. 2-Methylimidazole acts like a match that lights the hardening process of epoxy resins. Without a good hardener, an epoxy stay sticky and soft. By introducing this small nitrogen-containing molecule, manufacturers control setting time and physical strength. It unlocks extra durability for electronics, giving circuit boards the resilience to survive temperature changes and harsh environments. This matters in everything from city bridges to smartphones. I’ve seen repair crews curse brittle, easy-to-crack old plastics, but parts treated with upgraded epoxy mixtures keep doing their job.
R&D laboratories working on new medicines make use of many building blocks, and 2-Methylimidazole often steps in during synthesis. It doesn't end up in the pill bottle, but it keeps the process moving smoothly. Its core structure gives chemists a highway for building more complex molecules, including antifungals and some cancer drugs. Give a chemist 2-Methylimidazole and they can make molecules bend and connect in a hundred different ways. In the world of drug discovery, flexibility like this can mean faster breakthroughs and more affordable treatments.
Agriculture companies also rely on 2-Methylimidazole, especially through its role in producing certain pesticides and fungicides. Farms worldwide fight an endless battle against pests, and this molecule helps turn basic chemicals into protective agents. It advances the performance of crop treatments, raising yields and protecting the harvest when conditions get rough. Anyone who’s invested in a crop knows how crucial each percentage point in yield can be. Better protection for crops means fewer spikes in produce prices.
No chemical comes without baggage. 2-Methylimidazole works well, but studies show it can cause toxic effects if mishandled. Factory workers and researchers must handle it carefully, wearing protective gear and following tight protocols. Governments have set short-term exposure limits, but more data on long-term effects would ease some worries. Increased transparency around where and how these chemicals enter our goods would help everyone, not just those working in labs. Scrutiny pushes companies to design safer manufacturing lines and find even greener, less hazardous hardeners or reaction boosters as alternatives.
As consumers, we don’t often get a vote about what compounds build our goods. Pushing for stricter regulation and open data can steer manufacturers toward safer practices. Some companies already seek bio-based or lower-toxicity alternatives, but the real breakthrough will happen when safety and performance can truly coexist. Until then, 2-Methylimidazole continues to shape industries that shape society itself—quietly, but with real impact.
Handling chemicals means making choices that get personal fast. One such chemical, 2-Methylimidazole, seems routine in research circles, but it quickly proves that warnings on the label aren’t just there for show. It doesn’t matter if you spend most days hovering near beakers or only come across this stuff reading safety data sheets. The rules stay the same: don’t mess around with your protection. Nitrile gloves, splash goggles, and a lab coat aren’t decoration—everyone’s skin is different, but this material can irritate even the least sensitive hands. Absorbing it through the skin isn’t some chemistry myth; it actually happens. I’ve seen folks shrug off the gloves one day and nurse redness for the rest of the week.
Working with 2-Methylimidazole in a closed-up, crowded space? It doesn't take a chemical engineer to know that’s risky. Vapors build up quick in low ventilation labs, giving you headaches, sore throats, or worse. I’ve noticed people only think about fume hoods after someone starts coughing. Staying proactive by using a well-functioning fume hood every time keeps the air clear. Even a mask offers only part of the solution; rely on the right airflow from the start. Poor air quality sneaks up; nobody wants an invisible hazard floating around.
Lab benches are busy places, and accidents happen. Spilling 2-Methylimidazole feels like a minor hassle—until it isn’t. It’s tempting to grab some paper towels or splash on water, but swift, smart action works better. Scoop up the solid gently, using tools that don’t just spread it around. If it touches the skin, straight to the sink for a thorough rinse. Don’t improvise with cleaning solutions; stick with water and approved absorbents. I’ve learned it makes a huge difference to prep a real spill kit before anything ever slips from the bottle.
Tucking away a chemical at the end of the day seems routine, but a quick glance at chemical incompatibility charts could save a whole lot of trouble. Water, oxidizers, and acids are bad neighbors for 2-Methylimidazole. Stashing it away from moisture and sunlight helps keep its properties stable. One time, I saw a half-open container left out; the mess that followed was a lesson for everyone about mold, weird smells, and ruined samples. Regular labeling and organizing shelves isn’t bureaucracy, it’s prevention.
Handling this material gets personal. Even strong warning signs can get ignored once folks get comfortable. I’ve seen rashes, headaches, and eye trouble that don’t get chalked up to 2-Methylimidazole until people connect the dots. Eye wash stations and emergency showers simplify the recovery after contact—if you actually know where they are. Quick action right after exposure often turns a potential health scare into a lesson learned, nothing worse.
Sometimes, people only ask questions once something’s gone wrong. Turning that around starts with peer support. Safety training sessions let everyone share real problems and fixes—they reach past rulebooks into useful advice. Keeping chemical safety sheets close at hand gives you answers at your fingertips. If the instructions don’t make sense, or hazards go unmentioned, telling supervisors and getting answers immediately should be normal, not awkward. These efforts go way beyond just following rules; they keep work and learning going smoothly, and everyone goes home healthy at the end of the day.
Chemicals never leave your side when you spend your work days in a lab. 2-Methylimidazole? That’s one of those unsung heroes. Its chemical formula, C4H6N2, looks simple, but seeing those letters and numbers means plenty to anyone working with pharmaceuticals, coatings, or catalysts. Pulling out the periodic table, tallying up each atom, you land on a molecular weight of 82.11 g/mol. People might throw these values around, but anyone who’s ever needed to calculate a chemical reaction yield or mix a buffer knows exactly how vital these numbers are.The formula isn’t just classroom trivia. Whether you’re synthesizing drug molecules or making specialty epoxy resins for industrial use, you keep C4H6N2 in your notebook, your inventory software, and your head. Without nailing that molecular weight, calculations get messy fast. Errors in stoichiometry can stall a whole day’s work or burn through precious resources. Ask any graduate student who mislabeled their sample in a late-night frenzy—these details stick with you.
It’s tempting to gloss over chemical details. Plenty of fields, though, rely on people giving them serious thought. In pharmaceuticals, a tiny deviation during synthesis can create impurities, safety risks, or simply waste time and money. For coatings and polymers, 2-Methylimidazole ends up as a curing agent. The balance has to be right for the end product to perform as expected, especially under stress or in extreme environments. Over the years, I’ve seen new chemists flinch from asking a senior tech for the “right numbers,” but missing them can throw off bigger projects in production and testing.I remember a manufacturing push for high-performance resins. The plant manager started buying cheaper, off-brand materials with unclear documentation. The wrong molecular weight led to a failed batch—and suddenly dozens of people had to pull overtime to fix it. That single misstep came straight from not checking the exact chemical information and trusting a supplier sheet that looked “close enough.” Product recalls and safety hazards won’t wait for second chances.
This stuff has no real shortcuts. Every chemist, engineer, and technician needs reliable information at their fingertips. Reliance on battered copies of chemical handbooks or second-hand advice can’t keep up with real-world demands. Digitizing records, adopting verified inventory databases, and making sure every new tech gets real training on interpreting chemical data brings down the risk.Suppliers can support this by sharing detailed material safety data sheets (MSDS) that highlight formula and molecular weight up front. In-house lab meetings that encourage staff to double-check figures or flag uncertainties really make a difference, even if it slows things down by a minute. Working environments become safer, mistakes shrink, and product quality handles real scrutiny.For folks starting out, never assume a number or formula. Check and re-check. Working with something as straightforward as 2-Methylimidazole, the details define success far more than flashy new tech or big investments. In a world loaded with chemicals and competitive industries, precision isn’t optional—it’s how you earn trust, stay safe, and get out the door on time.
2-Methylimidazole sounds like a fancy chemical, but break it down and you’re basically looking at a powder that comes with its own set of headaches. Leave the safety data sheets to the compliance folks—everyday handling depends on knowing what’s in front of you. This is a substance that’s flammable and can cause real trouble if left around without any thought. I’ve seen people act casual with chemicals at labs, treating containers like they’re bags of flour, but things turn south fast when you forget what you’re dealing with.
If you leave 2-Methylimidazole out in a damp or warm spot, you’re inviting problems. It absorbs moisture from the air, which means any careless storage turns it from an easy powder into a sticky mess. I once saw a storage mistake where the air conditioning went out over a long weekend and someone left the chemicals in a room that got hot and stuffy. Opening certain jars later was like peeling open a forgotten bag of marshmallows in summer—clumpy, sticky, and unusable. Keep it dry, keep it at room temperature, and stay away from direct sunlight. Simple rules, but people cut corners if there’s no clear routine.
It’s tempting to save space by piling chemicals wherever you can fit them, but this is where trouble starts. 2-Methylimidazole shouldn’t sit beside strong oxidizers, acids, or bases. A little spill, a mix-up in closing the lids, and things react—sometimes with heat, sometimes with nasty smells, always with disruption. The smartest setups I’ve seen have clear labeling and keep this compound on its own shelf, not squeezed between unrelated products. Space is tight in most labs and storerooms, but shoving chemicals in together just piles up the risk.
Even with the best storage, someone’s got to grab the jar eventually. That’s usually a tech or student who’s thinking about the next step, not the dangers lurking in the air. Dust from 2-Methylimidazole floats—if you don’t handle it with gloves and a mask, your skin and lungs get the worst of it. In places where safety gear sits on a dusty shelf, people start skipping steps. Making it easy to access gloves, goggles, and masks helps everyone remember: you’re not just dealing with powder, you’re dealing with something that can do real harm if you’re lazy or distracted.
From what I’ve seen, mistakes don’t often come from not knowing the rules—they come from not building habits. Chemicals like 2-Methylimidazole don’t wait for you to remember the morning safety briefing. Setting clear routines, training new staff by showing (not just telling), and checking storage areas weekly can cut out most problems. Automated logs and alarms for improper temperature or humidity go even further. People say routines get boring, but boring doesn’t start a fire or ruin a whole batch.
Some folks get tempted by shortcuts, thinking they’ll just keep an eye on things. But 2-Methylimidazole doesn’t care about your good intentions. Shove it in the wrong place, ignore the humidity, forget to clean up spills, and you’re setting yourself up for headaches—or worse. Storing this chemical properly doesn’t have to become a science project. It only takes common sense, a bit of planning, and the will to do small things right every time. That’s how you keep work running smooth and everybody safe.
Anyone who has walked through a chemical storeroom or worked with purchasing at a manufacturer knows packaging can make or break a day’s productivity. The details matter. With a compound like 2-Methylimidazole, packaging goes past convenience—it has real consequences for safety, cost, and storage headaches.
Typical buyers in labs or industry run into 2-Methylimidazole offered in several types of packaging. For small-scale use, suppliers stock bottles around 100 grams and 500 grams. Most research work happens at this level, and those that handle small amounts appreciate the ability to open, use, and reseal with minimal fuss. These bottles usually come from brown glass, giving some light protection for a chemical that prefers to stay out of the sun.
Larger users look for 1 kg and 5 kg wide-mouth containers—usually sturdy HDPE or polypropylene. These plastic containers take a beating better than glass and stop moisture from creeping in. Factories and contract manufacturing lines jump up to bags, often 25 kilograms. These bags have liners that push out moisture and contamination risk. A pallet stacked with these bags saves money per kilo and fits existing automation systems for unloading and batching. I’ve seen businesses store weeks of raw material safely this way while keeping costs in check.
Regulations keep changing. Now, almost every supplier double-checks chemical handling standards for hazardous materials. Those bulk bags need proper hazard labels, tamper-proof seals, and sometimes UN-rated drums—especially if they cross borders. In a busy workplace, the right label avoids disaster. I remember a near miss from a mislabelled drum; no one wants a repeat.
I’ve noticed some buyers get stuck with oversize packaging that never matches real needs. If you’re running a small pilot or specialty chemical line, a 25 kg sack ends up half-open, absorbing humidity or spilling precious product. Smaller HDPE jugs let you portion out what you need, then keep the rest safe. For others, “splitting” shipments into practical sizes saves hassle even if it adds a few cents per kilo up front.
Waste adds up quickly. Single-use bags or bottles create piles of trash. Some chemical divisions work with suppliers to return plastic drums for reuse or recycling. A local plant I visited set up a closed-loop drum program and cut their disposal costs in half. This kind of partnership turns a problem into a practical win.
Labs favoring sustainability persuade their vendors to switch over to bulk refill systems or deposit-return drums. It’s not only big companies pushing for this. Even university labs choose suppliers that cut down on excess packaging—which helps budgets and the planet without extra policy meetings.
At the end of the day, matching packaging size to your real operation matters. It means less waste, fewer accidents, and a more efficient workflow. Chemistry is tricky enough; getting the right container for 2-Methylimidazole keeps things running smoothly and safely. That’s real peace of mind.
| Names | |
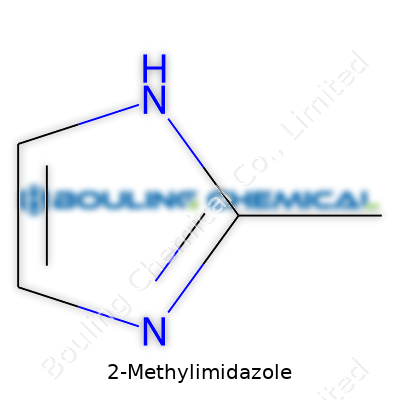
| Preferred IUPAC name | 2-Methyl-1H-imidazole |
| Other names |
2-Methylimidazol 2-Methyl-1H-imidazole 2-MI 2-Emid 2-Methylglyoxaline |
| Pronunciation | /tuː ˌmɛθ.ɪl.ɪˈmɪd.əˌzɒl/ |
| Identifiers | |
| CAS Number | 693-98-1 |
| Beilstein Reference | 27364 |
| ChEBI | CHEBI:34758 |
| ChEMBL | CHEMBL1438 |
| ChemSpider | 6737 |
| DrugBank | DB02197 |
| ECHA InfoCard | 06b8fd88-b6c1-484b-8696-64fd07e3fa1b |
| EC Number | 202-506-9 |
| Gmelin Reference | 73655 |
| KEGG | C06584 |
| MeSH | D013731 |
| PubChem CID | 700 |
| RTECS number | MK1400000 |
| UNII | D1J37QWA9L |
| UN number | UN3263 |
| CompTox Dashboard (EPA) | 7C2M2D8I8A |
| Properties | |
| Chemical formula | C4H6N2 |
| Molar mass | 82.11 g/mol |
| Appearance | white to pale yellow crystalline powder |
| Odor | Odorless |
| Density | 1.03 g/mL at 25 °C (lit.) |
| Solubility in water | soluble |
| log P | 0.02 |
| Vapor pressure | 0.0025 mmHg (25°C) |
| Acidity (pKa) | 14.5 |
| Basicity (pKb) | 7.48 |
| Magnetic susceptibility (χ) | -6.3×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.493 |
| Viscosity | 1.64 mPa·s (at 25 °C) |
| Dipole moment | 3.67 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 122.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -52.49 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3908 kJ/mol |
| Pharmacology | |
| ATC code | V03AB37 |
| Hazards | |
| GHS labelling | GHS02,GHS05,GHS07,GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P270, P280, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-3-0-** |
| Flash point | 85°C |
| Autoignition temperature | 470 °C |
| Explosive limits | Explosive limits: 2.9–19% |
| Lethal dose or concentration | LD50 Oral Rat 1300 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 970 mg/kg |
| NIOSH | NIOSH: RW2450000 |
| PEL (Permissible) | PEL: 5 mg/m³ |
| REL (Recommended) | ≤0.02 mg/m³ |
| IDLH (Immediate danger) | 900 mg/m3 |
| Related compounds | |
| Related compounds |
Imidazole 1-Methylimidazole 4-Methylimidazole 2-Ethylimidazole 2-Propylimidazole |