Turning back the clock to the early part of the 20th century, chemists happened upon a branch of compounds that later would shape both food science and pharmaceuticals. 2-Methyl pyrazine made its debut in the lab scene as people explored the aromas of roasted grains and beans. Researchers began isolating a series of pyrazines, noting how these compounds packed a punch in flavor and scent. It soon turned out that 2-methyl pyrazine appeared consistently in the results from toasty processes. Laboratories and universities fueled interest as they sought to understand flavors and offshoots in industrial chemicals. Its utility grew as the food industry matured, leading to broader commercial production by the 1970s, once synthesis methods became efficient and cost-effective. Major manufacturers caught on, stoking growth and regular use in all sorts of foods and corridor-research.
2-Methyl pyrazine usually hits the market as a clear, colorless to pale yellow liquid with a noticeable, nutty odor. Producers deliver it as a pure ingredient, dialing in consistency batch after batch. Food companies, flavor houses, and even pharmaceutical labs put it to use. It doesn’t take much to deliver the sharp, characteristic aroma people associate with roasted coffee, cereals, and even chocolate. Despite coming from labs now, it matches what nature delivers during Maillard reactions in cooking.
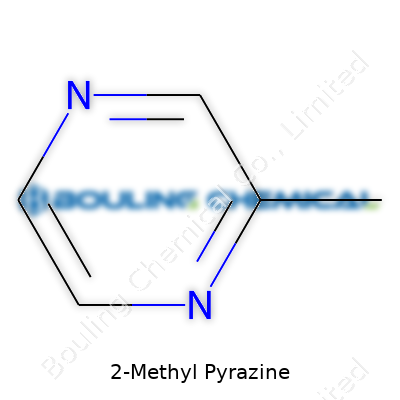
This compound’s chemical formula reads C5H6N2, placing it among pyrazines with a methyl group on the second carbon. Standing out with a boiling point around 139°C, it mixes easily with alcohol and slightly with water. Odor thresholds rest low, which means minuscule amounts can alter flavors. Its density clocks in a little under one gram per cubic centimeter at room temperature, making it manageable in standard facility settings. The molecular weight lands at about 94.12 g/mol. On the shelf, it resists light degradation more reliably than some similar compounds, though best practice keeps it away from direct sunshine and sources of ignition for both quality and safety.
When big suppliers ship 2-methyl pyrazine, the label carries a lot of details—purity typically exceeds 98%. Batch numbers, CAS registration (109-08-0), hazard codes, and recommended storage conditions all show up per global shipping standards. Regulations require labels to warn against ingestion and skin contact, reflecting its classification as an irritant. Companies also keep tabs on recommended usage rates, especially for applications in food, generally staying below ten parts per million. Efforts in quality control track impurities like other methylated pyrazines, water content, and residual solvents from the synthesis route.
Labs usually make 2-methyl pyrazine by cyclizing acetonin with ammonia, triggering a reaction that forms the pyrazine core. More advanced methods involve catalytic dehydrogenation of 2-methylpiperazine, which tightens output and lets large-volume plants keep costs lower. These routes cut down byproducts and waste compared to older, less targeted methods. Companies keep refining the reagents and design of the process to boost yields and keep energy footprints manageable. As demand grows, greener chemistry looks for paths using bio-based feedstocks or enzymes for a lower environmental load.
Chemists often view 2-methyl pyrazine as a building block for bigger molecules. Halogenation tacks on chlorine or bromine groups to set up pharmaceutical intermediates. Oxidation reactions target the methyl group, creating carboxylic acid versions for testing new drugs or agrochemicals. Alkylations and acylations on the ring allow for a staggering array of tastes and fragrances in new flavor development. Research labs sometimes couple this pyrazine with other aromatic rings, hunting for better therapies or more stable food ingredients. Its chemical backbone resists a lot of breakdown under mild conditions, letting formulators count on reliability whenever the supply chain brings it in.
A scan across chemical catalogs shows that 2-methyl pyrazine goes by a handful of names. Among them, the most common are alpha-methylpyrazine and methylpyrazine. The CAS number 109-08-0 keeps order across systems, flagging this specific molecule no matter what local language or supplier document you see. Marketing sometimes upsells it as a “coffee-roast enhancer” or “nutty flavor generator” in food industry terms, but the molecule underneath stays unchanged, no matter what label or technical sheet accompanies the product to the end user.
Working around 2-methyl pyrazine calls for a steady hand and adherence to proper safety procedures. This compound irritates the eyes, skin, and throat, and can provoke coughing or headaches if concentrations spike in the air. Personal protective equipment—gloves, goggles, lab coats—remains non-negotiable across all labs and plant floors. Material safety data sheets point out routes of exposure to avoid and list the need for proper ventilation. OSHA and related agencies in other countries set strict exposure limits, pushing companies to upgrade their extraction hoods and monitor room concentrations. Spills clean up with absorbent material but always under a hood or outdoors, away from ignition sources. Training extends to all users, not just chemists, making sure protocols stick no matter how routine a task seems.
Food science relies on 2-methyl pyrazine for that distinct, roasted touch in everything from breakfast cereals to plant-based meats. It brings the scent of popcorn, bread crust, and browned nuts, letting mass-market products mimic or amplify flavors without the cost of long roasting times. Beyond food, this compound forms part of some pharmaceutical manufacturing routes, especially in heterocyclic drug intermediates. Agriculture applications pop up, too, as new generations of crop protection agents demand novel pyrazine derivatives. Electronic noses and analytical chemistry devices pick up 2-methyl pyrazine as an environmental marker—tracing roasting activities, fires, or even illegal production in clandestine facilities. Its versatility outpaces many other molecules from the same chemical family, which rarely see so broad a spread between industries.
Labs worldwide pour funds into exploring new uses for 2-methyl pyrazine and its relatives. Application work in food tech designs new combinations that cut costs or replace allergens. In synthetic chemistry, tweaking the ring or methyl group leads to next-generation scents and flavors or unlocks pharma-grade reagents. Researchers test catalysts that handle this pyrazine at lower temperatures, hoping energy savings can add up at industrial scale. Green chemistry tries to sidestep fossil starting materials altogether by turning plant matter into precursor molecules with engineered microbes. Scientific conferences devote sessions to novel bio-flavor generation, bringing closer the day artificial and “natural” versions fall into alignment. Industry partners watch these developments for tools to keep their supply chains sustainable and consumer-safe, especially as regulations tighten and labeling laws change.
Toxicologists do not take 2-methyl pyrazine for granted. Controlled studies in lab animals point toward low acute toxicity by ingestion or inhalation at the doses usually encountered in food, but respiratory and skin irritation remains a concern at higher levels. Regulators like the US FDA and the European Food Safety Authority track new data closely, setting limits far below those where any real-world adverse effects cropped up in studies. Metabolite screening brings an extra layer of caution, as safety researchers check not just for direct effects but also for breakdown products in the body or environment. Scientists work to close gaps in chronic exposure findings, since new food uses ramp up annual tonnage and push for longer-term surveillance. Precaution wins out, and no production or flavor application skips a full hazard assessment before hitting commercial scale.
As technology evolves, 2-methyl pyrazine stands to play a bigger role in food innovation, health, and environmental monitoring. Consumer demand for plant-based and cleaner-labeled products lifts demand, as flavor designers turn to this molecule to replicate the roasted notes of traditional proteins and grains. Digital agriculture opens up chances to track soil and air molecules, using pyrazines as biomarkers for changes in farming or land use. Advances in enzyme engineering mean factories may soon tap renewable starting materials, lowering footprints for large-batch production. On the health front, new research into pyrazine modifications could yield advances in cancer, neurology, and infection-control drugs, as medicinal chemists look beyond classic ring systems for fresh leads. All the while, stricter compliance for safety and traceability pushes manufacturers to step up transparency and invest in better testing. Childhood memories of morning coffee or hot popcorn probably owe something to this compound—just with a lot more science behind the curtain than anyone realized at snack time.
Step into a bakery, lift the lid on a jar of roasted coffee, walk into a chocolate factory—there’s a good chance you’ll bump into 2-Methyl Pyrazine, even if you’ve never heard its name before. This chemical wears many hats, showing up in foods, fragrances, and even some unexpected corners of industry. It brings a distinct roasted, nutty aroma that feels as familiar as toast at breakfast or popcorn at the movies. For years, food scientists and flavorists have relied on it to punch up the taste and smell of snacks, cereals, and even pet food. Rather than relying solely on natural roasting, big companies use 2-Methyl Pyrazine to guarantee consistency in flavor no matter the batch or season.
I got my first real glimpse of how much work goes into processed snacks while doing college research in a food chemistry lab. Noticing that ingredients lists almost always included “artificial flavors,” I learned about substances like 2-Methyl Pyrazine. Companies add it to potato chips, crackers, and other packaged treats because it is stable, predictable, and affordable. A smart move, since flavors from natural roasting can swing depending on humidity, farm conditions, or even how long something sits on the shelf. With this pyrazine, you always get the same effect: a toasty, earthy punch that brings processed foods a little closer to the real thing.
Coffee shops and chocolatiers don’t stray far from this molecule, either. Roasting coffee or cocoa beans naturally releases a cocktail of pyrazines, with the methyl kind often topping the list. This gives your cup of coffee that “freshly brewed” scent and helps chocolate taste deeper. What’s interesting is that lots of “chocolate” or “roasted nut” flavored snacks rely on synthetic blends, sometimes using more pure 2-Methyl Pyrazine than you’d ever find in nature. The jury’s still out on whether this counts as “unhealthy,” but regulators have generally marked it as safe for consumption when used in small amounts.
The story doesn’t stop at snacks and coffee. Fragrance manufacturers use 2-Methyl Pyrazine to build woody, nutty bases for perfumes and air fresheners. Industrial labs grab it as a chemical building block, crafting more complex molecules. Even in pest control, you might find traces of it in bait designed to lure rodents, borrowing that irresistible nutty scent.
But the main pull comes from food. Since about 95% of artificial flavors trace back to a handful of such molecules, 2-Methyl Pyrazine sticks out. It finds its way into commercial kitchens and factories instead of homes because concentrated amounts can overwhelm and even trigger allergic reactions. Most home cooks won’t ever buy a vial of pure 2-Methyl Pyrazine. Instead, they’ll enjoy its results in breakfast cereals, microwave meals, or coffee creamers without ever knowing. Food scientists have to walk a careful line, studying safe exposure limits and keeping blends precise to avoid unexpected side effects or off-flavors.
For those thinking about what lands on their dinner table, understanding chemicals like this opens up a deeper conversation about processed food. Real transparency would mean clearer labels about source and purpose, not just “artificial flavoring.” If you’re after foods closer to the source, sticking with simple, recognizable ingredients reduces the chance of encountering additives like 2-Methyl Pyrazine in high amounts. For folks with allergies or sensitivities, it helps to ask brands about their flavoring process.
Food makers could do a better job of involving consumers in these choices. If more people asked about flavor chemistry, we’d see bigger pushes for honest labeling, maybe even safer or more sustainable production. Until then, 2-Methyl Pyrazine will keep doing heavy lifting in snack aisles and factories alike—sometimes right under our noses.
Some names in chemistry come with more weight outside the lab than folks imagine. Mention 2-Methyl Pyrazine and, to most, it sounds like a mouthful only chemists need to swallow. Strip away the jargon, and it all circles back to a simple pair of identifiers: its formula and its CAS number. The chemical formula for 2-Methyl Pyrazine is C5H6N2, giving us a tally of five carbons, six hydrogens, and two nitrogens. The unique CAS number assigned to this molecule is 109-08-0. Think of the CAS number as sort of like the bar code you’d find on a can of soup—each one singular, no duplications, making searches and sourcing that much easier.
Back in my college days, the aroma of roasting coffee and chocolate factories drifted over campus. Some afternoons it felt like catching comfort in a breeze, no matter how soulless the exam schedule. What I didn’t realize then? 2-Methyl Pyrazine quietly took part in those smells. It stands as a key flavor and aroma molecule, naturally forming during roasting, grilling, or even aging. Food and beverage companies keep close tabs on compounds like this, because a pinch added into a recipe can tip blandness into boldness or make fake cheese taste a bit closer to the real deal.
For people outside labs or production lines, chemical identifiers serve more than just cataloging ingredients. Allergies, regulatory compliance, and authenticity demands push folks to pay sharper attention. Someone who wrestles with sensitivities benefits from knowing what goes into packaged foods. The same details keep companies honest, making ingredient lists transparent so food scientists can dodge copycat confusion or legal headaches. Trading in only brand names and colorful package claims, you miss the traceability that a chemical formula or CAS number provides.
On the job, I once watched a mix-up halt a warehouse operation. Two drums looked the same—labels faded, batch stickers knocked off—but only one carried 2-Methyl Pyrazine, the other something entirely different. The chemical formula (C5H6N2) and CAS number (109-08-0) saved the day. The crew hit the database, matched the codes, and sidestepped a possible nightmare. This scenario crops up in manufacturing and distribution—one slip, a wrong blend, then product recalls or worse.
A lot of people hate the tedium of chemical bookkeeping, but digitizing these records and making public access simple would cut mistakes and boost trust. Consumer apps scanning labels could pull up these identifiers and tell users not just what’s inside, but which supplier made it and how safe it is. Farms to factories would face fewer counterfeiting risks. Chemical education in schools could tie these identifiers to real-world uses—so kids remember beyond tests and see what chemistry looks like at the dinner table or corner café.
A formula such as C5H6N2 or a CAS number like 109-08-0 looks sterile at first glance. In reality, knowing what these codes mean pulls back the curtain not only on lab work, but on the flavors and safety woven throughout daily life. Accuracy starts with calling things by their real name—even the names only a chemist could love.
You glance at the back of a crispy rice snack, see "2-Methyl Pyrazine," and wonder if that name belongs in a chemistry lab or your kitchen. It’s a common flavoring, used to mimic roasted, nutty, or baked notes. You’ll find it sprinkled throughout the world of processed food—cereals, coffee flavors, or even chocolate treats. For food scientists, it’s a small but useful tool for creating or enhancing familiar tastes.
Looking at any additive, safety breaks down to two big questions: is the compound toxic at the amounts we eat, and does it build up in the body over time? Multiple food authorities, from the US Food and Drug Administration to the European Food Safety Authority, have cleared 2-Methyl Pyrazine for use under specific conditions. Studies on animals show it has a low toxicity profile when used in tiny amounts—exactly the way food makers use it.
I spent years working in kitchens and talking with food production folks. Most flavorings get added by the milligram, not the spoonful. Toxic effects tend to show up only if a person downs massive amounts, far more than a single bag of chips could deliver. The Joint FAO/WHO Expert Committee on Food Additives draws safety lines through rigorous testing and keeps an ongoing eye out for new data. If new research flagged even a small issue, recall bells would ring loud.
People sometimes feel suspicious about ingredients with an unfamiliar name. The label “artificial” makes heads turn, but 2-Methyl Pyrazine actually occurs naturally in roasted foods. The stuff in factories usually gets made through chemistry, but the flavor and structure match what happens in your oven at home. Fears about “fake” ingredients often lead back to a lack of context or knee-jerk reactions to names that sound harsh.
Just like salt or caffeine, small amounts rarely cause trouble, but problems pop up in huge doses. There’s no reason to ignore long ingredient lists, though. Total exposure from everyday eating matters. That’s why watchdog groups ask for better food labeling and more research every year. Some people react to trace additives by avoiding processed foods entirely, but most of us do better with solid facts than fear-driven decisions.
Manufacturers hold the responsibility to use flavorings with care, and regulatory bodies should keep pushing for up-to-date science. Making sure ingredient levels stay well within safe ranges protects everyone. There’s nothing wrong with questioning what goes into food—scrutiny leads to better safety standards. Pushing for independent research, transparent ingredient lists, and educating people about what’s really on their plate goes a long way.
It’s easy to get lost in the alphabet soup of modern food labeling. Focusing on clear science instead of scary-sounding names gives us more control, not less, over what we choose to eat.
2-Methyl pyrazine turns up in flavor labs, chemical plants, and warehouses. It's known for its roasted, nutty aroma that nothing else quite matches. The compound sounds friendly enough, but handling it doesn’t reward carelessness.
Chemicals like to react, and 2-methyl pyrazine doesn't shrug off heat or direct light. I remember standing in a warehouse in August, watching a colleague stack drums right next to the loading bay’s sun-warmed wall. By noon, those containers felt hot to the touch. The catch: warmth speeds up unwanted reactions, sometimes leading to hazardous fumes or a leaky mess. A shaded, cool spot goes a long way toward extending shelf life and reducing incident calls at three in the morning. Keep it below 25°C, and sudden temperature swings don’t help either.
Leaving any chemical uncapped or using containers that don’t seal tightly is like hanging out laundry in a rainstorm—something unwanted will get in. Moisture can sneak inside, changing the nature of the product and inviting corrosion or strange odors. Good caps and solid containers block both air and water, keeping the contents pure. Glass or high-density polyethylene saves headaches down the road, especially when plastic jugs can react or deform.
You don’t need lab tests to notice a problem. A strong smell means something’s escaping. It's tempting to push leaky drums to the back, out of sight, but that turns a small issue into a big hazard. Spills near hot motors, forklifts, or packed pallets can lead to vapor clouds or, in the worst case, fires. Containment trays are not fancy extras. Floor-level collection pans and alert staff who know how to deal with drips help prevent a local house of horrors.
So much trouble starts when chemicals lose their name and date. Unmarked bottles become mysteries and get mishandled. Honest labeling gives everyone—down to the guy on the night shift—a fair shake at staying safe. Even simple details like “opened April 2024” tell the next person whether to use it or let it go.
By the book, 2-methyl pyrazine stings eyes and can irritate skin on contact. The bigger stories stem from mixing or storing it with strong oxidizers or acids. Once, somebody left it next to bleach—luckily, clear labels and a quick search stopped a potentially bad day. Keep flammable liquids and strong cleaning chemicals far apart; it’s not an old wives’ tale, it’s smart practice. Good ventilation—fans, open windows, or exhaust hoods—keeps stray vapors from settling at nose-level. Respirators and gloves don’t make you invincible, but they pile on peace of mind, especially if a splash or cloud catches you by surprise.
Reading a safety manual once won’t cement safe habits. Periodic walkthroughs, hands-on refreshers, and rewards for safe practice mean people actually follow procedure rather than guess. New hires and seasoned crews alike should know what’s in each drum, where to move it, and which problems demand a call for help. Investing in proper storage—climate control, leak-proof bins, real-time temperature tracking—beats the cost of chemical accidents any day.
A chemical’s risks are as real as the decisions made in the storeroom. From labeling to climate control, each choice adds a layer of safety. If rules seem tedious, remember: every minute spent on good storage and sensible handling saves hours lost to accidents, arguments, or ruined stock. I’ve never met anyone who regretted putting safety ahead of speed with 2-methyl pyrazine or anything like it.
Walking into a lab and spotting a bottle labeled “2-Methyl Pyrazine,” you might expect something dramatic because of the heavy name. Instead, you’ll find a clear liquid or a very faint yellow tinge, so pale it might escape notice if you aren’t looking for it. This isn’t one of those chemicals that surprises you with bright colors or a crystalline shine. The texture reminds me a bit of the way vegetable oil clings to glass, but without the viscosity. The label usually gives more away than the liquid sitting inside the bottle.
Back in my grad school days, I handled dozens of solvents and flavor compounds. Most looked about the same, but only a few gave themselves away by scent before a cap even came off. 2-Methyl Pyrazine stands in that group. If you spill even a drop, a faint yellow hue might show up on a white surface, but mostly it just leaves a sheen.
Odor sticks out much more than appearance. A person with no chemistry background could probably understand 2-Methyl Pyrazine’s importance after a single whiff. I’ve cracked open a bottle in workspaces and immediately caught the scent: something dry, warm, and slightly nutty, with an echo of roasted grains. That first hit reminds me of popcorn finishing up in the microwave, or the way a café smells if you walk in just as a new batch of beans gets ground. Some say the scent hints at chocolate, and I’d vouch for that, especially in the lingering tail end.
Pyrazines belong to a quirky family in the flavor world. You find their signature notes in snacks and cereals, and even cheap chocolate wrappers sometimes carry a trace. That roasted, bready warmth packs a punch even at low concentrations. Maybe that’s why flavorists chase after this molecule for coffee, bakery, and roasted nut flavorings. I remember working with food scientists eager to tune just the right trace of it into their product—sometimes too much added, the room would smell like an overdone croissant left in the oven.
People rarely pay close attention to what goes into engineered flavors, but compounds like 2-Methyl Pyrazine show up everywhere from beverages to chips. Its contribution to that comforting, roasty backdrop in food shouldn’t be dismissed. This is a classic example where chemistry jumps off the page of a textbook and lands right on your breakfast table.
Handling chemicals safely is a bit of a side note, but nobody should underestimate an aromatic like this. Even low concentrations can overpower a workspace. Not only does it tip off your senses, the compound lingers, clings to fabrics, and often has you catching phantom whiffs days later if you spill it. In production plants and food labs, the balance comes from exact measurement and solid ventilation, plus storage routines that keep everything tightly sealed to avoid cross-contamination.
Ever since I first worked with this molecule, I’ve respected its presence. Food scientists talk about it because it helps recreate a familiar comfort—think home-roasted peanuts or the steam when you open a bag of ready-made rice from the microwave. This single molecule highlights both the art and challenge in food science: keep the dose right, and you bring out the warmth people love; push too far, and you land an artificial, burnt aftertaste that no one wants to eat again.
That’s the balancing act everywhere I’ve seen it used—making something that feels natural and inviting, using a chemical that’s got so much character it needs careful handling. If you ever wonder why a snack tastes and smells just right, odds are someone understood exactly what 2-Methyl Pyrazine can do, and knew how not to let it take over.
| Names | |
| Preferred IUPAC name | 2-Methylpyrazine |
| Other names |
2-Methylpyrazine 2-Methyl-1,4-diazine Methylpyrazine Pyrazine, 2-methyl- |
| Pronunciation | /tuː ˈmɛθ.ɪl paɪˈreɪziːn/ |
| Identifiers | |
| CAS Number | 109-08-0 |
| 3D model (JSmol) | `3DStructure;JSmol;C1=NC=CN=C1C` |
| Beilstein Reference | 0227287 |
| ChEBI | CHEBI:28585 |
| ChEMBL | CHEMBL15414 |
| ChemSpider | 10149 |
| DrugBank | DB02254 |
| ECHA InfoCard | ECHA InfoCard: 100.012.740 |
| EC Number | 211-025-3 |
| Gmelin Reference | 7177 |
| KEGG | C06425 |
| MeSH | D011653 |
| PubChem CID | 13492 |
| RTECS number | XU3150000 |
| UNII | L81H70BQUN |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C5H6N2 |
| Molar mass | 94.12 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | nutty, roasted, cocoa |
| Density | 0.981 g/cm3 |
| Solubility in water | soluble |
| log P | 0.13 |
| Vapor pressure | 0.7 mmHg (at 25 °C) |
| Acidity (pKa) | pKa = 2.6 |
| Basicity (pKb) | 0.80 |
| Magnetic susceptibility (χ) | -41.0e-6 cm³/mol |
| Refractive index (nD) | 1.497 |
| Viscosity | 0.886 mPa·s (at 25 °C) |
| Dipole moment | 1.93 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | S°₍₂₉₈₎ = 274.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 67.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3376.9 kJ/mol |
| Pharmacology | |
| ATC code | V03AX |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: H302-Harmful if swallowed. H319-Causes serious eye irritation. |
| Precautionary statements | P261, P264, P271, P273, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 68 °C (154 °F; 341 K) |
| Autoignition temperature | 420 °C |
| Explosive limits | 2.6% - 19.6% |
| Lethal dose or concentration | LD50 oral rat 1,780 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 820 mg/kg |
| NIOSH | VV7525000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 1 mg/L |
| Related compounds | |
| Related compounds |
2,3-Dimethylpyrazine 2,5-Dimethylpyrazine 2,6-Dimethylpyrazine 3-Methylpyrazine Pyrazine |