Chemists searching for effective nitroimidazoles came across 2-Methyl-5-Nitroimidazole in the twentieth century, following the surge of interest in antibacterial and antiprotozoal agents after World War II. Laboratories in Eastern Europe and later across Asia started developing nitroimidazole derivatives as a response to rising infectious diseases. The success of metronidazole in the 1960s pushed other similar molecules into experimental pipelines, not just for infection control but also as tools to study drug action in anaerobic environments. 2-Methyl-5-Nitroimidazole’s journey started in this scientific climate, as researchers mapped modifications on the imidazole core to fine-tune biological activity and find something unique outside of the metronidazole shadow.
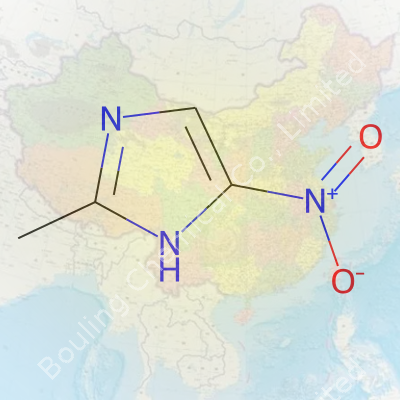
You walk into a chemical storage lab and spot 2-Methyl-5-Nitroimidazole on the inventory list. Its name reveals a simple backbone: an imidazole ring, methyl group at position 2, nitro group at position 5. That structure places it squarely among nitroimidazoles, valued for reactivity and proven biological punch. Pharmacologists take a close look at it for antiparasitic and antimicrobial screenings. Industrial chemists keep it on hand, since the compound shows up in intermediate steps on the way to synthesizing other medical agents. Chemical companies ship it as a fine, yellowish powder, often in tightly sealed bottles to limit atmospheric exposure.
2-Methyl-5-Nitroimidazole crystalizes as a light yellow powder. It dissolves freely in polar solvents like DMSO but only sparingly in water, a property that always shapes your handling decisions. Chemists measure its melting point at around 170–173°C, relying on those numbers as a marker of purity. Its molecular formula stands as C4H5N3O2 and it weighs in at 127.1 g/mol. The nitro group sits on that ring, drawing electron density away and setting the stage for both the compound’s chemical behavior and biological effects. You can pick up a faint, aromatic scent, but nothing overwhelming. Plenty of labs follow standard safety protocols, as nitro compounds warrant respect for reactivity—heat, friction, or strong reducing agents can spark tricky scenarios.
Labeling on a fresh batch includes the compound name, batch number, net mass, and warnings about skin, eye, and respiratory hazards. Technical sheets detail purity, often 98% or higher, along with melting point range and IR or NMR spectrum peaks for QC checks. Risk pictograms mark out its potential health or environmental effects. Safety data sheets in every proper facility flag the need for gloves, goggles, and adequate ventilation. It’s clear from the labeling that this isn’t a casual chemical, despite its routine role in the lab.
Commercial preparation generally starts from 2-methylimidazole—made by cyclization from glyoxal, ammonia, and acetaldehyde—followed by selective nitration. Nitration isn’t a trivial step: the process demands cold conditions, controlled acid addition, and careful temperature monitoring to avoid runaway reactions or unwanted byproducts. Labs scaling up the process set up recirculating cooling and add concentrated nitric acid dropwise. Washing, neutralizing, and recrystallizing yield the target nitroimidazole, with purity refinements dialing in the final product.
The nitro group doesn’t just provide biological activity—it also opens doors for new chemical modifications. Reduction can convert the nitro to an amine, leading to active pharmaceutical candidates or dyes. Some chemists swap out the methyl group for bulkier side chains, changing solubility or targeting. The imidazole ring handles acids and bases well but will react with strong nucleophiles or oxidizing agents, giving researchers a toolkit for both pathway construction and degradation studies. Tinkering with this structure often leads to analogs with just enough change to inspire a fresh round of activity tests.
You’ll hear more than one name for it. It shows up as 2-Methyl-5-Nitroimidazole on most datasheets, but older chemical catalogs may use methyl-2-nitro-5-imidazole. Some pharmaceutical patents call it MNZ for shorthand, though this can also refer to metronidazole, so local context matters. In certain regional markets, distributors swap in coded labels or proprietary names to flag high-purity material or specific grades, mainly for research rather than clinical use.
Lab techs pop open the container only after suiting up, since fine powder plus nitro group spells inhalation and reactivity risks. Safety standards follow international guidelines: fume hood work, nitrile gloves, splash goggles. Waste gets funneled separately for nitroorganics, never down main drains. Storage means a cool, dry place, away from direct light and incompatible materials like reducing agents or strong bases. MSDS sheets stay close by, and every spill, no matter how minor, gets flagged for environmental documentation. These standards stem not just from regulatory mandates but also years of hard-earned lab experience, where even minor lapses have caused accidents.
In industry, pharma labs use 2-Methyl-5-Nitroimidazole as a starter for medicinal chemistry programs aimed at treating protozoal and bacterial infections. Beyond drugs, it plays a part in agricultural research where nitroimidazoles disrupt parasite metabolism in livestock. Some analytical chemists even tap into the compound’s electron-drawing nitro group as a probe for redox chemistry. Universities rely on it for teaching nitration and reduction reactions to undergrads, who always ask about its real-world bioactivity. Whenever a new imidazole analog appears promising, you find researchers retracing steps to this versatile building block.
Research journals still publish work leveraging 2-Methyl-5-Nitroimidazole for drug analog design. Structure-activity relationship studies line up dozens of related molecules, tracking changes in antiparasitic or antimicrobial potency. In fermentation optimization, microbiologists tweak environmental factors to see how the compound fares against anaerobic pathogens like Trichomonas or Giardia. Some teams have explored radiolabeling it for tracer work, taking advantage of the imidazole’s metabolic uptake in hypoxic tissues or tumors. Its versatility shows up every time scientists try to outsmart resistant microbes, and the compound still attracts funding where resistance overruns older therapies.
Toxicologists monitor exposure carefully. Older animal studies found that in high doses, the compound—like its cousins—can cause neurotoxicity or tissue irritation. Metabolic breakdown often generates reactive intermediates, which means chronic exposure sits under tight scrutiny. Researchers check for genotoxicity and reproductive effects, flagging the nitro group for its links to DNA interaction. Modern labs keep exposure far below the no-observed-adverse-effect level (NOAEL) and integrate hazard controls that include skin protection and prompt cleanup. Environmental teams on the production side study breakdown rates, since nitroimidazoles in wastewater can persist unless targeted by advanced treatment plants.
Antimicrobial resistance doesn’t pause, and neither does the search for smarter drugs. Med chem teams probe new substitutions on the imidazole ring, hoping to sidestep resistance mechanisms and toxic side effects. Environmental scientists put pressure on suppliers to green up industrial synthesis, searching for milder nitration agents or recyclable solvents. Computational chemists use 2-Methyl-5-Nitroimidazole as a testing ground for machine-learning models trying to predict new biological hits. At teaching institutions, students get an early lesson in combining caution with chemical curiosity thanks to this compound’s profile, and new applications keep trickling down from research to pilots faster than many would predict.
2-Methyl-5-Nitroimidazole doesn’t roll off the tongue, but its history carries weight in medicine. This molecule forms part of a family known as nitroimidazoles, a group of compounds that doctors have counted on for decades. If you check the ingredient list for drugs targeting stubborn infections, you’ll sometimes find a name that looks similar. The best-known sibling in this chemical family is metronidazole. It’s found in flagyl, a medication that’s been saving lives in clinics and hospitals for years, especially across places facing limited healthcare supplies.
Drugs made from 2-Methyl-5-Nitroimidazole knock out bacteria and single-celled parasites. They don’t go after every bug, only ones that grow best without oxygen—like the critters causing giardiasis, trichomoniasis, and certain gut infections. Metronidazole, for example, fights off Clostridium difficile (C. diff), a nasty germ that often pops up after a person takes too many antibiotics. Doctors also reach for this class of drugs to treat dental abscesses, pelvic infections, and some forms of meningitis.
Modern medicine is always rushing forward. New drugs appear every year, each one claiming to be safer, faster, or broader in its reach. Still, you find nitroimidazoles like 2-Methyl-5-Nitroimidazole in medical kits around the world. In many places, people can’t afford fancy treatments. These older drugs work, cost less, and often save lives when nothing else is available. In my own work at a community clinic, patients come in after trying home remedies for infections. Giving them a proven treatment—usually metronidazole or similar—turns drawn-out sickness into quick relief. The smiles and simple thank-yous from those patients remind me that dependable medicine beats a long sales pitch about the next wonder drug.
Easy access brings trouble, too. Overuse of drugs based on 2-Methyl-5-Nitroimidazole has triggered resistance in some bacteria and parasites. The world saw this with malaria, too. Smart bugs and parasites adapt fast, outsmarting the medicines that once stopped them cold. Resistance leads to longer treatment, higher costs, and more suffering. In crowded hospitals where infections can hop from patient to patient, the problem spreads quickly. Poorly trained health workers, fake medications on the market, and self-medication only add fuel.
Clinics and health departments face a tough puzzle. On one side, they depend on affordable, effective treatments. On the other, they risk running out of options if drug resistance keeps climbing. Careful prescribing helps. So does patient education—making sure people finish their course of treatment, don’t share pills, and check with licensed professionals before picking up antibiotics. Governments and organizations should monitor resistance. Investing in basic lab testing lets clinics see if a particular bug will respond to nitroimidazoles, or if it’s time to try something else. No one wants to sit by while yesterday’s cure becomes tomorrow’s missed chance.
The world keeps changing, but some challenges stick around. 2-Methyl-5-Nitroimidazole and its chemical cousins offer a good example of why practical, affordable treatments matter. As long as people need help fighting infections, medicines like this still have a job to do—if we take care to use them wisely.
Walking into a lab and seeing a jar labeled 2-Methyl-5-Nitroimidazole, the first impression comes from its pale yellow or sometimes light brown crystalline powder. You pick up a vial, tap it, and notice it doesn't clump up much. Every chemist appreciates a compound that stays dry and pours easily. It handles a lot like other nitroimidazoles, so people familiar with related molecules expect similar behavior.
If you heat it, those small crystals start to melt somewhere around 122°C to 125°C. Stick your nose closer and catch a slightly musty, chemical odor; nothing sharp or fruity here. Its modest solubility in water — not much more than a couple grams per liter — defines most of its handling, especially for people mixing up solutions or prepping samples.
You pour it into ethanol or methanol and watch it dissolve much more eagerly. That fact alone steers technicians to use alcohol-based solvents in the lab for dissolving, cleaning, or analysis. If you spill granules on a bench, a quick wipe is enough, since the powder doesn’t stick around the way finer dusts do. Too much humidity can be a problem, but it doesn’t pull moisture from the air like some salts.
The nitro group at the 5-position punches up its chemical reactivity, setting it apart from imidazole neighbors without nitro substituents. That one little group opens the door to both chemical reduction and substitution reactions. Anyone running an organic synthesis will notice that the nitro group acts as a major handle, making this compound a versatile intermediate. Specialists in pharmaceutical research recognize this instantly — nitroimidazoles have a reputation for antibacterial and antiprotozoal action.
Some folks worry about nitro compounds for good reason. Under reducing conditions, that nitro group can become an amine or other functional groups, changing the molecule’s whole personality. The methyl group at position 2 does more than just bulk things up; it alters how the compound stacks and bonds with other chemicals, sometimes tweaking potency or selectivity if this molecule winds up in a drug.
Stored in a cool, dark place, 2-Methyl-5-Nitroimidazole keeps well. Light and heat, over time, eat away at its stability. High temperatures speed up decomposition. If someone leaves a bottle open, there's a risk for slow degradation, so containers need a solid cap and a dry shelf.
Take precautions, but don’t panic: this isn’t a compound that jumps at the chance to explode or burn. Its main risk comes from dust or powder in the air. Inhaling nitroimidazoles over the long haul, especially in a production setting, isn’t smart. Gloves and masks become routine for good reason.
Labs have a few tricks to make life easier. Use sealed sample vials, limit the quantity on open benches, and rely on fume hoods if any hot work is needed. Training new staff about the specific quirks and safety routines with nitroimidazoles avoids mishaps. Manufacturers could offer smaller pre-weighed doses in sealed containers, cutting down on handling steps and reducing spills. Even small shifts in safety culture go a long way when dealing with active nitro compounds daily.
In my time around research labs, I’ve learned that every compound brings its own set of handling challenges, and 2-Methyl-5-Nitroimidazole is one of those that demands respect. This pale-yellow powder comes with volatility—both in chemistry and in storage musts. It isn’t just another bottle you can stash in any cabinet. Instead, think of it like a houseguest with specific dietary needs. Ignore those requirements, and you’re likely to invite trouble.
2-Methyl-5-Nitroimidazole wants shelter from humidity and direct sunlight. Heat, mistakes with airflow, or even slight moisture can spoil its integrity and create risk. My own practice taught me that keeping such chemicals in well-sealed, airtight containers is a basic act of respect for everyone’s safety. I’ve seen instances where careless storage led to clumped powder, degraded product, and potential safety scares.
Temperature tells another story. Cool, stable environments suit this compound best. If you’re thinking about tossing it next to a radiator or in a crowded, sunbathed room, you might as well speed up its decomposition. A dry and cool chemical storage cabinet, away from heat vents or any ignition sources, works best. I’ve always trusted cabinets with built-in thermometers for peace of mind.
Nitroimidazoles, in general, don’t mess around when it comes to sensitization or toxicity. If you work around them long enough, even a mild spill or a careless touch can teach harsh lessons. Wearing gloves isn’t optional; neither is donning safety goggles. People sometimes forget the irritation potential on skin or eyes until they feel it firsthand. Lab coats and fume hoods are real friends here. My colleagues have stories of respirable dust kicking up during weighing. Taking the few seconds to work under a vent is always worth it. Even tiny particles count, and ordinary cloth masks don’t cut it. Go for a mask rated for chemical exposure, and change it regularly.
Accidents do happen. Once, in a poorly equipped teaching lab, I saw a minor spill on a benchtop. The student reached for paper towels, and the powder ended up in the air, drifting around. A vacuum with a HEPA filter and wet cleaning technique would have stopped the spread, but only proper training will create that instinct.
For fire risk, dry powder extinguishers belong nearby. Storing anything flammable or reactive in the same area does more harm than good. In my experience, fire drills that give everyone muscle memory save the day if something goes south. Emergency showers, eyewash stations, gloves, goggles, lab coats—it’s not just about ticking boxes, it’s about people walking out unharmed.
I’ve seen the difference simple checklists and regular training make. Label containers clearly, review your chemical inventory often, and keep the Material Safety Data Sheet within arm’s reach, not in a locked drawer down the hall. Communicate with coworkers, train new staff personally, and never cut corners on protection. Good habits build safe labs, and safe labs protect everyone’s future.
You don’t find 2-Methyl-5-Nitroimidazole in the shopping aisles or the medicine cabinet. This compound usually comes up in labs, sometimes in discussions about pharmaceutical precursors or specialty chemicals. It’s not widely known, but the question of whether it’s hazardous or toxic matters to those handling it.
Chemical names tend to sound intimidating. From experience, I’ve seen how researchers don lab coats and goggles the moment nitroimidazoles show up in their inventory. These steps aren’t for show. Compounds in this family sometimes end up in medications to fight off bacteria or parasites. Metronidazole, for example, has a solid track record in hospitals. But a small tweak in molecular structure can sometimes spell big changes in risk.
2-Methyl-5-Nitroimidazole isn’t likely to land in a child’s lunchbox, but in chemical workspaces, exposure can happen in ways most folks don’t realize. This compound carries a nitro group—an arrangement known in chemistry for producing little bursts of toxic activity inside the body. These aren’t feel-good fireworks; nitro groups can break down and release free radicals that bash through DNA and cell structures. Anyone who’s worked with nitroaromatic or nitroheterocyclic compounds knows gloves don’t just protect from stains.
Studies run on similar nitroimidazoles, including in laboratories overseas, have turned up signs of mutagenicity—basically, these chemicals sometimes cause little typos in genetic material. That’s bad news, especially in workspaces with regular exposure. It’s true that 2-Methyl-5-Nitroimidazole hasn’t had the massive safety reviews reserved for popular drugs, but by comparing its relatives, it’s clear why caution kicks in.
Toxic effects often depend not just on the molecule but on the dose and the route—swallowed, inhaled, or spilled on the skin. It doesn’t take much imagination to picture a splash during mixing or a tiny cloud of dust during transfer. Without proper precautions, these accidents can set the stage for headaches, dizziness, skin irritation, or worse.
Rather than play guessing games, folks who handle this stuff treat it with respect. Safety data sheets point out that nitroimidazoles, particularly those with modifications like methyl and nitro groups, can trigger toxic responses in lab animals and possibly humans. This isn’t a surprise for chemists—there’s a reason fume hoods hum all day in the lab.
Even so, chemistry isn’t the enemy. It’s the lack of respect for these risks that causes headaches—sometimes literally. Once, helping with a reaction involving a related compound, I watched as someone shrugged off the need for a face mask. They powered through, got a nose full of dust, and spent the afternoon regretting it with a pounding headache. Sometimes the body offers its own warning system.
Training and clear labels help people avoid trouble. Gear like gloves, goggles, and ventilated hoods aren’t up for negotiation in a lab that deals with 2-Methyl-5-Nitroimidazole. Disposal routines should never mean dumping it down the sink. Most sites ship hazardous waste off for incineration or specialized treatment.
Regulators in countries with strong chemical safety rules keep close tabs on substances like this one. If any workplace wants to avoid health problems or fines, following those rules just makes sense. For now, every person using 2-Methyl-5-Nitroimidazole has a choice: handle it with care, or risk learning about toxicity the hard way.
Chemicals with a name like 2-Methyl-5-Nitroimidazole sound like something hidden deep in an industrial catalog. But this compound shows up on lab benches for a reason—its performance in reactions relies on one key factor: purity. 2-Methyl-5-Nitroimidazole mostly lands in inventories with a purity tipping over 98%. High-purity material helps researchers trust their results, especially in pharmaceutical and chemical synthesis. I’ve seen batch-to-batch consistency make or break a project. Lingering impurities muddy up reactions, leading to unpredictable yields or side products, which means wasted time and resources. Those problems amplify when the compound gets used as an intermediate for more complex drugs. For many scientists, a certificate saying “≥98%” signals confidence, but a few suppliers push purity closer to 99%, mainly for more technical applications.
Walking through any chemical storeroom, the right package saves headaches, both in storage and safety. 2-Methyl-5-Nitroimidazole usually gets shipped in a handful of standard sizes. My own lab has pulled it from small amber glass bottles, often in 25-gram or 100-gram amounts—these work for most research needs and cut down on waste if you only need a few grams per experiment. Firms that handle bigger batches or run multiple reactions go for 500-gram bottles, or even full kilogram plastic drums. With kilos, you see differences in packaging style. Smaller glass bottles block out UV and keep things dry, while polymer drums do better for short-term storage in bulk, as long as humidity is low and the storeroom isn't a sauna.
Anyone handling fines like this should take packaging seriously. The chemical’s powdery form spreads, and even a small spill leaves a powerful yellow cling on gloves and benches. Proper sealing is not a formality—good packaging protects both product quality and the safety of technicians. My habit has been to open new bottles inside a fume hood, since even light dust has a knack for getting airborne. Larger batches stored in drums come with desiccant packets packed inside liners to stop any moisture uptake. The little things matter: tight caps, thick liners, and knowing you have the right PPE before breaking the seal.
In pharmaceutical development, no one wants stories of contamination penalties or project delays from a mislabeled batch. If the starting chemical isn’t pure, the next steps become a guessing game. I’ve heard colleagues talk about chasing down weird chromatogram spikes, only to track the culprit to leftover impurities in a supposedly pure supply. Researchers order sizes that match their run rate—not just to save cash, but also because leftover opened bottles pose risks as they age. Too many half-used chemicals linger until someone runs a quality check, spots a problem, and then you’re scrambling to replace stock.
Straight talk: no one outside the lab worries about the purity numbers on a drum. But inside, it’s everything. Small-scale labs stick with 25 or 100 grams. Production areas shift to kilos, but only with the right sealed packaging and documentation. Reliable supply chains help—especially today, with global shipping unpredictability. Good suppliers keep their purity reports visible and stick to formats that labs actually use, plain and simple.
For any group facing inconsistent supplies or worrying about purity drifts, sourcing from certified vendors with good feedback is a start. Keeping track of unused bottles and using first-in, first-out inventory helps avoid aging stock. On the supplier side, clear lot documentation, robust security seals, and smart packaging choices remove a lot of headaches. In tight lab spaces, compact bottles matter just as much as purity numbers.

| Names | |
| Preferred IUPAC name | 2-methyl-5-nitro-1H-imidazole |
| Other names |
Metronidazole 1-(2-Hydroxyethyl)-2-methyl-5-nitroimidazole 2-Methyl-5-nitro-1H-imidazole |
| Pronunciation | /tuː ˈmɛθɪl faɪv ˈnaɪtroʊ ɪˈmɪd.əˌzoʊl/ |
| Identifiers | |
| CAS Number | [694-83-7] |
| Beilstein Reference | 106875 |
| ChEBI | CHEBI:48582 |
| ChEMBL | CHEMBL1547 |
| ChemSpider | 198470 |
| DrugBank | DB00916 |
| ECHA InfoCard | ECHA InfoCard: 100.008.573 |
| EC Number | 214-909-4 |
| Gmelin Reference | 718015 |
| KEGG | C07347 |
| MeSH | D008875 |
| PubChem CID | 15618 |
| RTECS number | NI8225000 |
| UNII | Q3672QAG4M |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID3020184 |
| Properties | |
| Chemical formula | C4H5N3O2 |
| Molar mass | 129.11 g/mol |
| Appearance | Light yellow crystalline powder |
| Odor | Odorless |
| Density | 1.29 g/cm³ |
| Solubility in water | Soluble in water |
| log P | 0.02 |
| Vapor pressure | 0.0000154 mmHg at 25°C |
| Acidity (pKa) | 8.61 |
| Basicity (pKb) | 11.89 |
| Magnetic susceptibility (χ) | -41.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5180 |
| Viscosity | 1.36 cP (20°C) |
| Dipole moment | 4.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 285.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -17 kJ mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -308.8 kJ/mol |
| Pharmacology | |
| ATC code | J01XD03 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P270, P273, P301+P312, P330, P501 |
| Flash point | 103°C |
| Lethal dose or concentration | LD50 oral rat 1440 mg/kg |
| LD50 (median dose) | LD50 (median dose) = 1300 mg/kg (rat, oral) |
| NIOSH | RN 99-80-9 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.05 mg/m³ |
| Related compounds | |
| Related compounds |
Metronidazole Tinidazole Ornidazole Secnidazole Dimetridazole |