Back in the middle of the twentieth century, researchers looking for more powerful antimicrobial compounds turned their attention to imidazole derivatives. The addition of a methyl group at the second carbon and a nitro group at the fifth position led to a molecule both intriguing and practical. This marked a major step for chemistry and medicine, since scientists realized these structural tweaks directly influenced biological activity. Over decades, 2-Methyl-5-Nitro Imidazole earned attention as labs and pharmaceutical makers sought alternatives to more traditional treatments for protozoal and anaerobic bacterial infections. When resistance started creeping into households and hospitals, this compound’s roots became important for development strategies aiming to outpace bacterial adaptation.
2-Methyl-5-Nitro Imidazole shows up in labs as a pale yellow crystalline powder. Chemical suppliers often stock it for research, pharmaceutical processes, and intermediate use in synthesis. Its growth as a staple of microbiological testing and drug development can’t be separated from the demand for safer, effective agents to treat infections beyond the reach of simple penicillins or cephalosporins. Across the world, its availability has fueled new pharmaceutical syntheses and multiple generic product launches. Its unique placement among nitroimidazoles also supports unusual or hard-to-treat infection strategies, giving clinicians new tools against some stubborn pathogens.
This substance carries a molecular weight hovering around 155.13 g/mol and displays a melting point between 185 and 190°C. It resists easy dissolution in water yet blends smoothly with common organic solvents such as ethanol or dimethyl sulfoxide. It carries a slightly bitter odor, rarely strong enough to notice outside of pure form handling. The molecule holds tightly to its aromatic ring, with electron-rich nitrogen and oxygen atoms lending themselves to hydrogen bonding—something that shapes its behavior in both lab reactions and biological environments. Sensitivity to intense light and oxidative conditions means storage choices often focus on dry, cool, and dark environments.
Laboratories must check for purity above 98 percent by HPLC or GC, with appearances rated regularly for color, consistency, and absence of foreign materials. Labels carry lot number, expiry date, country of origin, and manufacturer’s batch certifications for all clinical or research applications. Safety information, hazard pictograms, and recommended personal protective equipment show up at every purchasing level, from bulk shipment drums to research vials. Documentation standardizes around International Union of Pure and Applied Chemistry protocols and follows GO/No-GO criteria for active pharmaceutical ingredients, especially in regulated territories.
Production generally begins with methylimidazole as a base compound, subjected to a controlled nitration process. This step uses a mixture of concentrated nitric acid and sulfuric acid under low temperatures to discourage runaway byproducts. The nitro group lands at the five-position through direct substitution, with yields locked in through careful adjustment of temperature, acid concentration, and agitation speed. Industrial routes repeat these steps with closed reactors and in-line cooling to minimize risk. Purification often includes repeated crystallization using ethanol or a similar medium, followed by vacuum drying to remove trace reagents.
The presence of both nitro and methyl groups means this molecule behaves distinctively compared to parent imidazole. The nitro moiety enables reduction to the corresponding amine, a key move in drug discovery and custom molecule design. Methyl, meanwhile, boosts lipophilicity and shifts selectivity in biological pathways. Derivatization at the N1 or additional substitutions across the ring allow medicinal chemists to deepen antimicrobial activity or slice away toxic effects. In organic chemistry, 2-Methyl-5-Nitro Imidazole acts as both a target for further synthetic building and a probe for studying electron transport inside cells. Reports have described alkylations and acylations, revealing wider applications in agrochemical and dye research, not just as an antibiotic precursor.
Chemists call this compound by several other names: metronidazole intermediate, 2-Methyl-5-nitro-1H-imidazole, and 1H-Imidazole, 2-methyl-5-nitro. Trade and regulatory contexts may classify it under research numbers or internal company codes, depending on the place and intended application. Drug development teams and inventory controllers track these synonyms to align operational data, supply chain records, and regulatory registrations worldwide, since fragmented names could lead to delayed shipments or compliance gaps in multinational operations.
Despite a strong safety profile in formulated drugs, handling concentrated 2-Methyl-5-Nitro Imidazole demands respect inside laboratories and pilot plants. Long-term exposure to powders or dust can irritate the skin and airways, calling for disposable gloves, safety glasses, and effective ventilation systems. Material Safety Data Sheets specify emergency actions for spills, accidental ingestion, and fire containment. Regulatory guidelines such as REACH and OSHA spell out exposure limits and prescribe labeling for transportation and storage. Anyone involved in regular handling receives targeted training, keeping workplace safety audits and environmental controls air-tight where these chemicals flow.
This compound’s core action involves disrupting DNA synthesis in target organisms, a trait valuable for treating infections no longer responding to frontline antibiotics. It stands behind the clinical success of several antiprotozoal and antibacterial agents, including widely deployed nitroimidazole drugs like metronidazole and tinidazole. Beyond pharmaceuticals, researchers use it to illuminate pathways of microbial resistance and to create new test strains in microbiology. Industrial scale-ups have branched into production for veterinary drugs, environmental water testing agents, and plant protection blends where soil-borne pathogens threaten crop yields. Biotech pipelines see it as a springboard for building ever more selective anti-infective molecules.
Pharmaceutical innovators leverage 2-Methyl-5-Nitro Imidazole in screening programs designed to outmaneuver drug resistance, with teams focusing on new analogs that dodge emerging bacterial counter-mechanisms. Academic groups use it to model how nitro-containing drugs interact with human metabolic enzymes, hoping for breakthroughs in low-toxicity, high-potency drug forms. Synthesis and medicinal chemistry units automate reaction variations on its backbone to build focused libraries for testing in bacteria, protozoa, and even some fungal pathogens. Each new publication pushes understanding another step forward, leading to pipeline candidates in not just infection, but also inflammatory and oncological settings.
Scientists pay close attention to cellular and systemic safety, since nitroimidazoles have a checkered past regarding mutagenic and carcinogenic potential, especially under long-term or high-dose conditions. Rodent studies and cell assays map out metabolic breakdown products, revealing how even minor byproducts can trigger genotoxicity given sustained exposure. Toxicologists keep refining test models, factoring in not only direct human effects but also downstream impacts if the compound enters aquatic systems. Regulatory filings hinge on this research, as agencies demand proof that environmental, occupational, and clinical exposures stay well under harm thresholds defined by decades of animal and human data.
Antibiotic resistance shows no sign of slowing down, so this molecule’s place remains as crucial as ever. The flexibility built into its structure encourages new thinking: combination therapies, site-targeted prodrugs, and next-layer modifications to sidestep resistance entirely. Environmental chemists have started adapting it to degrade faster after use, softening impact on wastewater systems and wildlife. Automated synthesis and artificial intelligence screening offer the promise of dozens of novel analogs rolled out in less time and at lower cost. Partnerships spring up between academic labs, private industry, and healthcare stakeholders, all betting that future generations of nitroimidazole-derived compounds will continue fending off the world’s stubbornest infections while minimizing risk for people and planet.
Walking into a pharmacy, most people never hear about 2-Methyl-5-Nitro Imidazole. Still, it works quietly behind the scenes in lots of health solutions. This chemical, easy to overlook, forms part of some essential drugs that fight against parasites, bacteria, and stubborn infections. Its real strength lies in the nitro-imidazole group — a small setup of atoms that punches far above its weight against disease-causing organisms.
Doctors and researchers favor this compound for good reason. It’s not just the name that sounds technical. Its structure gives it flexibility and power. Drugmakers often build on its backbone to treat disorders that don’t budge with old-fashioned medicine. Think of serious intestinal or vaginal infections. In resource-limited hospitals, options often run thin, and these nitro-imidazole derivatives step in when affordable, reliable results matter most.
Why do scientists pick imidazole-based drugs? They work by damaging the DNA of bad microbes, stopping infection at its root. One study after another shows clear evidence. Anti-parasitic drugs like metronidazole rely on similar chemistry. By twisting parts of the basic imidazole ring and adding a nitro group, researchers have found ways to make treatments more targeted and potent.
I’ve sat through long training seminars with health workers in remote places, broken generators humming in the background, and watched them talk about drugs like these as lifelines. In areas where resistance spreads fast and options dwindle, tweaking just one part of a molecule can make the difference between hope and another hard day.
Resistance isn’t just a buzzword. The more doctors use a class of antibiotics, the faster bacteria learn to adapt. As a result, labs turn to molecules like 2-Methyl-5-Nitro Imidazole. Its relatively new status on the pharmaceutical scene gives it an edge against common culprits. Every new molecule gives doctors room to outmaneuver resistant bugs before they take hold.
Some concerns do come up. Handling any compound that can disrupt DNA means scientists stay on their toes about toxicity. Drug approval requires a mountain of safety tests. Governments keep lists of approved substances for a good reason. Manufacturing and transporting these chemicals takes experienced hands and a strong regulatory eye. Anyone dealing with them—lab techs, pharmacists, or shippers—relies on rules that keep people safe.
Even with good options on the table, the work never stops. As resistance grows and diseases pop up in new places, drug researchers push for even smarter versions of these molecules. Investing in research and encouraging open data sharing helps scientists learn from mistakes and build on success. Every year, teams in universities and private labs chase after tweaks and improvements, hoping to keep one step ahead of bacteria and parasites that refuse to quit.
No single compound can fight every infection forever, but the story of 2-Methyl-5-Nitro Imidazole shows how constant curiosity, close teamwork, and patient-centered science change lives in big and small ways.
2-Methyl-5-Nitro Imidazole isn’t some obscure entry on a lengthy chemical inventory. Its formula, C4H5N3O2, points to a compact arrangement of atoms that make a difference in both research labs and practical applications. Seeing these five carbon atoms, five hydrogens, three nitrogens, and two oxygens grouped together reminds me just how much can hide behind a short set of letters and numbers.
Every time someone in the sciences picks up a tube labeled 2-Methyl-5-Nitro Imidazole, they count on the accuracy of that formula. That six-syllable mouthful stands for more than just a molecular skeleton—it’s the gatekeeper for trust. In my time around chemical stocks in academic research, accurate idea of what’s inside each bottle saved time and stopped small mistakes from growing into bigger ones. Mislabeling happens less often now, but only because so many people keep an eye on formulas.
Take hospitals and pharmaceutical companies. Their chemists and pharmacists check, recheck, and sometimes pause to check again. 2-Methyl-5-Nitro Imidazole’s formula means everything if someone builds new drugs or studies resistance in microorganisms. It features the imidazole ring—a core part of medicines like metronidazole and related treatments. Smuggling an extra nitrogen or losing an oxygen changes everything: activity, toxicity, the way it breaks down in the body.
A friend who studied pharmaceutical chemistry told me about the time they caught a supplier mistake with nothing more than a calculator and a sharp pencil. An extra methyl group in the structure meant the whole order went back. That’s how it goes in science—one atom sends an experiment in the wrong direction.
Relying on published formulas, researchers don’t just toss information from a database onto a report. Solid data on C4H5N3O2 lets them predict solubility, flag hazards, and sketch out production plans. Miss a nitrogen and you get the wrong melting point. Add too many hydrogens and you throw a synthesis off. School chemistry classes love stoichiometry problems that rely on accuracy like this, but outside the classroom, whole research budgets can hinge on these numbers.
No one wants to base decisions on shaky details. Cheminformatics teams dig into old papers and modernize databases, because trusting a formula means tracing it back to reliable experiments. I once saw a professor dismiss a week’s worth of computational chemistry because someone copied a structure from an unreliable website. Multiply that by the number of research groups worldwide and it’s clear that formula accuracy shapes real progress.
Fact-checking C4H5N3O2 should come from more than one reputable source. Editorial boards, journal reviewers, and inventory managers build rigorous cross-referencing into their routine. Quality control teams in the chemical industry keep their jobs by treating verification as a daily habit, not a one-time event. As more people access digital resources, it will take a blend of vigilance, education, and technology to ensure that formulas stay reliable, accurate, and meaningful.
2-Methyl-5-nitro imidazole belongs to a group of chemicals used in pharmaceutical research and synthesis. It shows up as a yellow crystalline powder, and shows some promise in certain industrial or medical lab settings. In my experience working with between-the-lines chemicals, safety becomes a much bigger deal than folks might think at first glance.
I remember my early days in the lab, tracking powders by the scoopful and standing over reaction flasks. Looking at 2-methyl-5-nitro imidazole, it’s easy to underestimate risk just because it lacks a skull-and-crossbones label. But this compound carries some weighty hazards if you check the material safety data sheets. Breathing in dust might irritate lungs and nasal passages. Getting it on your hands or into your eyes can trigger redness or even burns.
There’s the nitro group sitting on this molecule. Chemistry veterans know nitro compounds sometimes spark off dangerous reactions. Under certain conditions, especially with heat or if mixed with other reactive substances, there’s the chance for more severe burns or even fire. Working in a university setting, I watched an overconfident co-worker mix a similar compound and spark an unexpected minor fire from nothing more than a static shock. Personal protective equipment saved fingers and eyesight that day.
If you breathe dust from 2-methyl-5-nitro imidazole, you might notice coughing or a scratchy throat. Contact with skin can bring on itchiness or lasting redness. The nitro structure suggests possible toxic effects for organs if exposure goes on over long periods. Some animal studies show certain nitro imidazole derivatives have ties to liver toxicity and suspected mutagenicity. Official data on this exact chemical runs thin, but smart safety practice relies on erring toward caution.
World standards often lag behind, so this compound rarely gets listed on short hazard lists. The lack of detailed toxicity data creates a false sense of security for some workers. The ECHA (European Chemicals Agency) and OSHA leans on recommendations for similar nitro compounds: gloves, goggles, good airflow, and disposal by licensed chemical waste handlers. My experience lines up. A little paranoia with personal safety pays off over a career spent surrounded by reactive powders.
Putting trust in lab coats, nitrile gloves, and safety goggles stands as the non-negotiable core of working with almost any chemical powder. Don’t forget proper ventilation—fume hoods strip potentially hazardous vapors and dust out of your breathing zone. Keep a spill kit and a clear path to the eyewash station 10 steps away. It helps to work with a buddy who notices if you get sloppy or distracted. Training isn’t just a box on a form. Reviewing emergency procedures every month might be irritating, but it turns panic into muscle memory if something actually goes wrong.
Labeling all containers and writing down every handling step matters, too. If contamination happens, quick cleanup using recommended solvents (not just water, which sometimes spreads the mess) limits harm. Thorough washing of hands and face before leaving the area is a daily ritual in every lab worth its salt. If symptoms appear after exposure—dizziness, rash, or lingering cough—a check-in with occupational health staff should follow.
Chemistry doesn’t always look dangerous from afar, but stories from the bench show risks hiding in plain sight. Ratings on hazard labels change over time as science catches up to real-world outcomes. Doubt keeps fingers attached and lungs unharmed. Lab safety isn’t about paranoia—it’s about making sure everyone who starts a project gets to finish it, too. 2-Methyl-5-nitro imidazole calls for respect, not fear, and cautious habits make every lab a little safer each day.
2-Methyl-5-Nitro Imidazole, a chemical with both laboratory and industrial relevance, doesn’t forgive casual treatment. Chemical stability hinges on storage. If this compound drifts even a little outside recommended storage parameters, you invite degradation and safety risks. My time handling reagents in busy university labs, and speaking with colleagues in pharmaceuticals, has made the costs of such errors clear—lost research time, wasted batches, and deeply annoyed safety officers.
Moisture always seems ready to ruin things, and with 2-Methyl-5-Nitro Imidazole, water exposure can drive hydrolysis or encourage clumping. That’s why a tightly sealed amber glass bottle finds its place here. Dry conditions matter, and standard desiccators work well for small quantities. Excess humidity or direct sunlight hues the powder or even changes how it reacts, which would throw off any subsequent synthesis or analysis. Many researchers wrap their containers in foil or tuck them away in dark cupboards—one more step, but simple and effective.
Room temperature in scientific terms—about 20–25°C—means something different than your home on a summer afternoon. Warm storage places can kick off slow decomposition, letting byproducts creep into your work. Refrigeration doesn’t suit every substance. For 2-Methyl-5-Nitro Imidazole, standard ambient temperature suffices, assuming it stays steady. Going below 0°C sometimes shocks certain compounds, causing condensation as the container is reopened and closed. I once kept a similar heterocyclic compound too close to the freezer coils—powder pulled in water, turned gummy, lost potency. Lesson learned.
So much comes down to clear labeling—date received, lot number, and expiration. As soon as original packaging disappears, mistakes take root, especially with lookalike jars on a cluttered shelf. On a team project, poorly marked substitution cost our group a week's worth of work and a pile of reagents. Good labels give every technician, supervisor, or inspector the info to make the right call, even two years down the line.
Handling nitroimidazoles raises toxicity concerns. Powders disperse with little trouble, and a lid left loose for a minute can send particles airborne. I still catch myself reaching for goggles and gloves, even just to weigh or transfer a scoop, after decades in research. Spills in storage areas can linger, contaminating future work—routine inspections and secondary containment trays reduce long-term headaches.
Nothing beats regular walk-throughs and inventory checks. It’s easy to let standards slip during a busy week, but overlooked containers can go unnoticed for months. Annual or biannual reviews of stored chemicals serve as preventive medicine. Automation helps, but even a simple logbook or an app notification system makes accountability stronger. Training newcomers thoroughly, showing rather than telling them storage details, creates habits that last. This quiet attention to proper storage stops expensive mistakes before they get a chance to grow.
2-Methyl-5-Nitro Imidazole falls into a cluster of specialized chemicals useful in labs and industry. Most buyers run into red tape before getting anywhere near this substance. While common solvents and lab supplies pop up on e-commerce sites with little friction, 2-Methyl-5-Nitro Imidazole rarely sits on those digital shelves. Smaller labs, university departments, and businesses plug into global chemical networks because that’s where the real supply comes from—and those networks check credentials before ink hits a purchase order.
This compound features in research, synthesis, and sometimes as a building block in pharmaceuticals. Some of its chemical relatives have wound up on regulatory lists due to dual-use risks—chemical precursors stir attention. Laws don’t target hobbyists out of spite; missteps can spark real danger.
Countries put in controls after seeing the fallout from chemicals leaking into the wrong hands. The U.S. Drug Enforcement Administration, European Medicines Agency, and similar groups in other countries clamp down on chemicals with the potential for harm. Sourcing chemicals legally keeps everyone above board.
A chemical like 2-Methyl-5-Nitro Imidazole does not show up at chain pharmacies or hardware stores. Specialized distributors such as Sigma-Aldrich, TCI, or Alfa Aesar have earned trust by keeping detailed records and operating within tight regulations. Before they ship anything, buyers submit documents. Proof of business status, lab licenses, end-user declarations—all form the backbone of a legitimate deal.
I’ve worked with labs that spent days filling out supplier registration forms, waiting out background checks, and responding to follow-up about how we planned to use a certain chemical. Direct contact with the supplier speeds things up compared to online shopping carts. Order delays on these products signal that a supplier follows the rules.
Some people get tempted by online “lab supply” marketplaces with anonymous vendors. That move carries real risk—not just legal trouble, but uncertainty about what you’re buying. Impure or mislabeled product throws a wrench into experiments or processes. Online forums occasionally attract buyers frustrated by the paperwork, but they can also attract scammers or worse.
Real chemical suppliers operate under government rules for storage, labeling, and record-keeping. They ship with proper customs paperwork and clear contact information. Spotting safety data sheets and lot numbers tells me I’m likely dealing with the real article.
Researchers and businesses go through registered distributors for a reason. It protects their own work, the people handling the product, and anyone downstream. It adds red tape, but that tape wraps up a system built after many hard lessons.
If a product listing skips verification and offers the chemical to anyone with a card number, alarm bells should go off. Labs working on tight deadlines sometimes get impatient with regulations, but those protections help keep the doors open and trust intact.
Navigating chemical procurement today comes with real hurdles, but streamlining account verification and improving transparency within the supply chain would help. Legitimate buyers benefit from supplier relationships; investing time up front pays off with better service, faster fulfillment, and fewer surprise audits down the road.
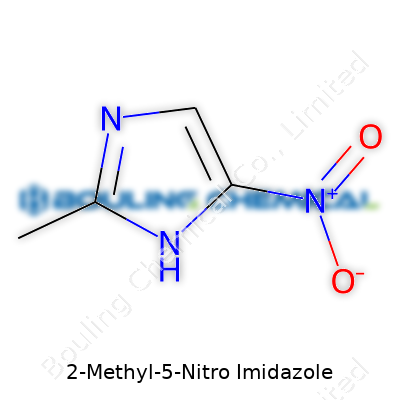
| Names | |
| Preferred IUPAC name | 2-methyl-5-nitro-1H-imidazole |
| Other names |
Dimetridazole 2-Methyl-5-nitroimidazole 1,2-Dimethyl-5-nitroimidazole |
| Pronunciation | /tuː ˈmɛθ.ɪl faɪv ˈnaɪ.trəʊ ˌɪm.ɪˈdəʊl/ |
| Identifiers | |
| CAS Number | 88054-22-2 |
| Beilstein Reference | 107770 |
| ChEBI | CHEBI:72838 |
| ChEMBL | CHEMBL1239 |
| ChemSpider | 43196 |
| DrugBank | DB00916 |
| ECHA InfoCard | 03d1dbfa-8e2e-45e5-970e-eb62edf45323 |
| EC Number | 613-515-7 |
| Gmelin Reference | 691376 |
| KEGG | C18733 |
| MeSH | D008069 |
| PubChem CID | 13943 |
| RTECS number | NL2975000 |
| UNII | QIT3VIM7Z8 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID9020702 |
| Properties | |
| Chemical formula | C4H5N3O2 |
| Molar mass | 129.10 g/mol |
| Appearance | Light yellow to yellow crystalline powder |
| Odor | Odorless |
| Density | 1.36 g/cm3 |
| Solubility in water | Soluble in water |
| log P | 0.01 |
| Vapor pressure | 9.3E-5 mmHg at 25°C |
| Acidity (pKa) | 8.61 |
| Basicity (pKb) | 7.53 |
| Magnetic susceptibility (χ) | -44.2e-6 cm³/mol |
| Refractive index (nD) | 1.579 |
| Dipole moment | 3.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 138.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -38.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2066 kJ/mol |
| Pharmacology | |
| ATC code | J01XD02 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H301, H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-1 |
| Flash point | 113.3 °C |
| Autoignition temperature | > 460 °C |
| Lethal dose or concentration | LD50 (oral, rat): 1300 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 1300 mg/kg |
| NIOSH | RG 384 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.05 mg/m³ |
| Related compounds | |
| Related compounds |
Metronidazole Tinidazole Secnidazole Ornidazole Dimetridazole |