Long before researchers began using imidazoles in pharmaceuticals, chemistry labs focused on modifying the basic imidazole ring to tweak biological activity. The search for new radiosensitizers and antimicrobial agents pushed scientists to look closely at nitroimidazole derivatives. Chemists first explored the nitroimidazole family in the 1960s, chasing answers to radiation resistance in tumors. Over decades, 2-Methyl-4-Nitroimidazole entered the conversation as a promising candidate when drug designers looked for compounds that offered potency and selectivity without the baggage of severe side effects. This compound owes its rise to the twin tracks of academic research and pharmaceutical demand, both aiming to balance chemical stability with medical potential. Publications from the 1970s started highlighting the advantages of methyl substitution at position 2, showing how it steered the molecule’s electronic properties and reactivity, an insight that still resonates in labs today.
2-Methyl-4-Nitroimidazole stands as a yellow to yellow-brown solid, meeting the benchmarks for intermediates in pharmaceutical production and research chemicals. Producers scale it for both gram and metric ton output, delivering purity suited for demanding synthesis applications. Its structure features a five-membered ring, bearing a methyl at the 2-position and a nitro group at the 4-position, allowing further derivatization to support different synthetic goals. Pure lots typically display a signature melting range and a strong nitro group absorbance under IR spectroscopy, making quality control straightforward. Many labs have adopted it for its balance between reactivity and manageability in storage and handling, so it stays a staple on chemical supplier lists.
The crystalline nature of this compound sets it apart from oily or hygroscopic nitroimidazoles. Melting occurs in the 120-130°C window, and the product preserves its structure under common storage conditions, away from light and moisture. The compound resists rapid decomposition, so it tolerates standard lab environments, which simplifies transportation and handling. A key trait, the nitro group at the 4-position, boosts electron-withdrawing effect and guides its role as both a mild oxidizer and a precursor for other synthetic steps. Methyl substitution on the ring increases its hydrophobic character, supporting bioavailability in medicinal uses and tuning its solubility between polar and nonpolar solvents. Solubility fits typical N-heterocycle profiles: decent in DMSO, dimethylformamide, and moderately in alcohols, with lower values in pure water.
Those purchasing from reputable suppliers find 2-Methyl-4-Nitroimidazole packaged in amber bottles or sealed bags, each batch accompanied by certificates of analysis. Identity is verified by high-performance liquid chromatography and mass spectrometry. Common specs demand purity well above 98%, with impurities such as parent imidazole or chloride salts held below strict thresholds. Labels carry CAS numbers, hazardous materials pictograms, batch numbers, and shelf-life information. The information supports both regulatory compliance and traceability across pharma, fine chemical, and academic users. Standardized documentation makes it easier for end-users to record, store, and retrieve lot data for future audits or safety reviews.
In the lab, synthesis usually follows a condensation between glyoxal and methylamine under controlled temperature, yielding a key 2-methylimidazole intermediate. Nitration proceeds with mixed acid systems—sulfuric and nitric acids—targeting selective substitution at the 4-position. Careful conditions ensure that nitration does not wander off to other carbons or ring nitrogens, requiring expert control of temperature and acid ratios. Following synthesis, purification relies on recrystallization or column chromatography. The process generates chemical waste that demands treatment, so most manufacturers implement green chemistry improvements, such as recycling acids or using less hazardous oxidants. Yields from modern protocols stretch over 60%, supporting both cost and sustainability goals. Advances in continuous flow chemistry hint that even greater efficiency and safety could soon become standard for this molecule.
In research, 2-Methyl-4-Nitroimidazole acts as a versatile scaffold. The electron-deficient nitro group encourages nucleophilic aromatic substitution, especially for amines or thiol moieties under basic conditions. Chemists transform the 4-nitro group to amine or hydroxyl derivatives through catalytic hydrogenation or reduction with tin(II) chloride. Other modifications tap into the methyl group, using bromination or oxidation to build further complexity into the structure, tailoring it for anti-protozoal or radiosensitizing properties. Cross-coupling possibilities also open up with proper functional group modifications, so new drug candidates or tracer molecules often grow out of this robust core.
Chemical catalogs list several names: 2-Methyl-4-Nitroimidazole, 2-Methyl-4-Nitro-1H-imidazole, and a variety of research codes used in drug development programs. Certain regional suppliers attach their own branding for market distinction, but all trace lineage back to the basic imidazole scaffold. Synonym lists matter because regulatory approvals and material safety data sheets often use different names. Whether a researcher in Tokyo or Frankfurt requests it, the core chemical identity stays consistent, supported by harmonized CAS indexing.
Exposure limits for 2-Methyl-4-Nitroimidazole rest on its nitroaromatic character and evidence from animal testing. Laboratory staff avoid inhalation and prolonged skin contact, with standard PPE such as gloves, coats, and splash goggles forming the routine barrier. It does not qualify as an acute inhalation risk but treats as a long-term toxic hazard, especially in case of skin absorption. Waste collection targets minimization of environmental discharge, and flammable material storage rules keep it away from oxidants and acids. Spill protocols use inert absorbents and closed disposal, so accidental exposure and environmental release rarely occur in professional labs. Emergency procedures include eye wash and shower stations, reflecting institutional commitment to lab worker safety.
In the field, 2-Methyl-4-Nitroimidazole finds a home among radiosensitizers used in cancer radiotherapy. Researchers prize it for its ability to mimic hypoxic cell environments, making malignant tissues more susceptible to radiation. This effect, noted in medical journals over decades, helped it carve out a niche in both clinical trials and preclinical models. Parasitology also turns to this molecule, searching for treatments against anaerobic bacteria and protozoan parasites. Veterinary medicine, crop protection, and polymer modification have all drawn on nitroimidazole chemistry at some point, giving this compound reach far beyond the hospital. The chemical also serves as a reference material in analytical studies and metabolism research.
Blood, sweat, and data have gone into pushing the boundaries of 2-Methyl-4-Nitroimidazole’s potential. Labs fine-tune analogs to chase safer and more potent radiosensitizers, aiming for selective action within tumor microenvironments. Studies gauge everything from enzyme binding to DNA repair disruption, in hopes of using structure-activity relationships to predict new hits. Machine learning now contributes, churning out thousands of virtual variants to screen before any real glassware touches a molecule. Open questions still surround mechanism in some biological settings, but clear gains emerge with each round of clinical or animal study. Pharma budgets flow into scale-up research, eyeing cost-effective and sustainable synthetic routes, which translates to more drug candidates entering late-stage testing every year.
Toxicologists scrutinize nitroimidazoles for mutagenicity and organ toxicity. 2-Methyl-4-Nitroimidazole shows low acute toxicity in rodents, with threshold limit values based on chronic study endpoints. Its metabolites and breakdown products receive careful attention, especially since nitroreduction in vivo can trigger undesired side effects. Environmental persistence presents another concern, so regulatory agencies monitor its use and disposal. Data from long-term occupational exposure surveys guide modern safety limits. In practice, reported clinical cases of major harm remain rare, provided that personal protective equipment and fume hoods carry the main burden during handling or reaction workups. Analytical chemists continue developing better detection assays, ensuring that even trace contamination in medical or agricultural samples can be stamped out before harm occurs.
Nature seldom gives up secrets easily, but as researchers learn more about the complexity of hypoxic cell biology and resistant parasites, 2-Methyl-4-Nitroimidazole sits poised to influence several scientific crossroads. Synthetic chemists push for greener processes, aiming to recycle solvents and catalysts, reduce carbon footprint, and improve atom economy. Medical application teams chase derivatives that show increasingly fine-tuned selectivity, helping patients tackle cancer and infectious disease with fewer side effects. Regulatory bodies and scientific organizations strengthen guidelines on safe handling and environmental stewardship. With these new pressures and the expanding toolbox of chemical modification techniques, 2-Methyl-4-Nitroimidazole remains a touchstone for creative research across pharmaceutical, agricultural, and materials science domains. Future breakthroughs likely hinge on both old-fashioned bench work and digital innovation, blending experience, theory, and new data streams to keep this compound part of tomorrow’s discoveries.
2-Methyl-4-nitroimidazole, or 2M4NI, tends to pop up most often in research circles. Not everyone has heard of it unless they have dabbled in chemistry, pharmacology, or oncology labs. Even so, understanding what it does sheds light on how behind-the-scenes chemicals shape medical progress.
Some scientists focus on designing drugs that boost cancer treatment. Tumors, especially those with hypoxic (low-oxygen) cores, often survive traditional therapies. 2M4NI helps researchers break through those barriers. It acts as a radiosensitizer—this means it makes tumor cells more sensitive to radiation. For patients, that opens the door to better results with lower radiation doses, which might spare healthy tissues from harsh side effects.
Studies dating back decades show nitroimidazole compounds like 2M4NI bind more strongly to cells low in oxygen. Fast-growing tumors often suffer from poor circulation, which means they carry those low-oxygen pockets. 2M4NI zeros in on those hard-to-treat zones, making radiation therapy more effective. In practice, this can help clinicians target stubborn cancer cells.
Labs have also tested 2M4NI outside cancer. Its molecular backbone shares features with anti-parasitic drugs used against infections such as giardiasis or trichomoniasis. Chemists often swap out parts of the molecule to see if related compounds work better or offer fewer side effects.
Research into 2M4NI goes further—scientists want to know how changes in structure affect the way these chemicals behave in the body. Some experiments suggest modifications could help fight bacteria that resist other drugs. Research is ongoing; many discoveries stay in preclinical stages, but the curiosity and effort keep moving forward.
Treatment resistance takes more lives than any single mistake in therapy. Friends and family members watch loved ones go through years of ups and downs. More tools in the toolbox mean more chances for hope. Compounds like 2M4NI don't always reach pharmacy shelves, yet without this kind of chemical groundwork, the next generation of treatment options never gets off the runway.
For anyone who has seen someone wrestle with cancer, the hunger for better treatments is more than academic. It's exhausting to watch traditional therapies hit a brick wall because cells hide in low-oxygen zones. Seeing talented teams push molecules like 2M4NI through the pipeline feels personal. Each new discovery brings a shot at extending life or making side effects less brutal.
The journey from promising molecule to approved medicine isn't easy. Researchers have to show that 2M4NI hits only tumor cells, doesn't linger dangerously in healthy tissues, and works well when combined with other drugs or radiation. They run into problems with toxicity, metabolism, and manufacturing costs. Good research answers tough questions: How do we keep patients safe? Can chemists make this stuff reliably and affordably? What happens in real-world use, not just in mice?
Stronger collaborations between academics, hospitals, and pharmaceutical companies could speed things up. More funding for early-phase trials and clearer rules for safety testing help researchers go from glassware to patient bedside. Chemists get creative, tweaking the molecule to lower side effects without losing punch. Doctors keep sharing insights from the front line so the next batch of drugs actually fits patient needs.
Looking back, every modern medicine has roots in fearless experimentation—and a bit of luck. 2M4NI plays a role in that ongoing story. Its value comes from lighting up pathways to better tumor targeting and finding new uses in the fight against infectious disease. With every study, the field steps closer to answers that could turn lives around.
The safety of any lab or production site relies on more than just good intentions. Chemicals can make life easier or a lot more dangerous, depending on how they're treated. 2-Methyl-4-nitroimidazole isn’t a common name in everyday conversation, but anyone working with it should give serious thought to how it’s stored. Mishandling isn’t just a paperwork issue—it can lead to exposure, accidents, or even ruin experiments. My years on the bench taught me that simple oversights grow into trouble faster than any spilled beaker.
2-Methyl-4-nitroimidazole holds its stability at room temperature, but that doesn’t give a green light to toss it anywhere. Storage in a cool, dry area is not just tradition—it cuts down on the risk of degradation or unwanted reactions. Excess heat can speed up decomposition, which may produce toxic fumes. I’ve seen unexpected humidity warp labels and carry moisture into containers. That’s why I always keep desiccants handy and make sure shelves aren’t near a window or heat source. Never trust an old air conditioner to protect your chemicals through a blazing July.
Sunlight streaming into a storage room may look cheerful, but it’s bad news for light-sensitive compounds. To protect 2-Methyl-4-nitroimidazole, use amber glass bottles or opaque containers. I once watched a colleague discover faded powder and failed results in a clear vial left on the top shelf. That small slip may sound minor, but research time and materials add up quickly. Always reseal containers firmly after each use, cutting off air and moisture before they have a chance to get inside.
Combining strong oxidizers with nitro compounds can be a recipe for more heat and even fire. 2-Methyl-4-nitroimidazole should not share a shelf or cabinet with acids, bases, or oxidizing agents. I organize storage by hazard class, not by how neatened up the cabinet looks. It takes effort, but I’ve seen the savings in terms of injuries avoided. Safety Data Sheets exist for a reason: read them, and don’t improvise.
Missing labels cost real money and sometimes even health. Always mark the date a bottle gets opened, and check containers routinely for leaks, cracks, or fading text. Good inventory makes it possible to spot trouble before it’s on your hands. Digital tracking systems work, but nothing beats walking the shelves with a checklist each month.
Wearing gloves, eye protection, and sometimes even a lab coat isn’t up for debate. Even outside direct handling, PPE shields against dust or incidental contact. Keep spill kits and neutralizing agents within reach. Never put chemicals close to food or drink, no matter how busy the day gets—cross-contamination can be subtle but dangerous.
Expired or unusable 2-Methyl-4-nitroimidazole shouldn’t end up in regular trash or poured down a drain. Chemical waste containers exist for a reason, and licensed disposal contractors handle the rest. I've learned that shortcuts in disposal come with fines or worse, so following disposal protocols isn't just about rules—it's about protecting staff, neighbors, and the environment.
Safe storage habits grow from a blend of training, clear procedures, and a little bit of stubbornness. If one person speaks up about a badly placed bottle, others take notice. Investing in checklists, periodic reviews, and solid storage materials helps keep chemicals like 2-Methyl-4-nitroimidazole from becoming a hazard. The best labs I’ve seen treat safety as part of working life, not a box to check after an inspection.
2-Methyl-4-nitroimidazole might sound like just another name in a lab notebook, but its impact reaches outside chemistry books. Used in research, pharmaceuticals, and sometimes environmental studies, this compound falls under the nitroimidazole group. These types of chemicals often play a role in drug development—sometimes as antibiotics, sometimes as experimental agents for cancer treatment.
Personal experience in a research lab taught me to respect chemicals with nitro groups. They often come with safety sheets flagged orange or red—caution is never optional. Those labels matter, and not just for folks in white coats.
Scientists do not slap a “hazardous” tag on a chemical just for kicks. They look at how it interacts with the body and the environment. 2-Methyl-4-nitroimidazole can trigger toxic effects if mishandled. Studies have raised red flags about its ability to damage organs if inhaled or swallowed and irritate skin or eyes on contact.
Similar nitroimidazoles have shown the ability to mess with DNA, which always raises questions about cancer risk or genetic harm. A nitro group, especially, likes to trigger metabolic reactions that can land someone in a hospital if they let their guard down. Decent evidence points to potential mutagenic effects in animal cell cultures, with some compounds from the same family linked to tumor growth. No one wants to take that gamble, whether working in a lab or living near a facility handling this stuff.
Research into compounds like this one has shown that even a few milligrams dumped into water systems can cause ripples. Nitroimidazoles can linger, affecting fish and other aquatic life. Water treatment plants struggle to break down these molecules. That means small leaks from factories or disposal accidents can stick around a lot longer than most folks realize.
Years working in environmental monitoring showed me that regulations don’t always keep up with emerging risks. Areas near industrial sites sometimes show higher traces of these chemicals in water and soil. Regular testing helps, but cleaning up after the fact proves much tougher than stopping pollution at the source.
Putting safety first means treating 2-methyl-4-nitroimidazole with respect. Labs keep it locked up and use special gloves, face protection, and solid ventilation. Everyday work with it demands routine safety checks; safety showers and eye wash stations become non-negotiable in any space where it shows up.
Companies and research groups who use this compound owe it to staff and the planet to prepare spill kits, label containers correctly, and train workers in real-life drills, not just after-the-fact PowerPoint slides. Where alternatives exist, companies look for less harmful substitutes. In some fields, switching out the compound or updating waste disposal systems helps keep both people and water safer.
Regulators keep reviewing chemicals like this one as new studies roll in. It helps when consumers and local communities keep an eye on reports about manufacturing practices. Anyone living near a plant or laboratory can ask about what’s being discharged and how waste gets handled.
People don’t have to handle chemicals directly to be affected by them. Open access to safety data, better spill reporting, and honest communication between labs, companies, and communities help keep problems from slipping through the cracks.
Knowledge gives power. Taking hazards seriously—even with a relatively obscure compound like 2-methyl-4-nitroimidazole—isn’t just smart chemistry; it’s common sense.
2-Methyl-4-nitroimidazole sure looks complicated as a name, but it breaks down easily with a little chemistry knowledge and curiosity. This compound, used in several chemical and biological applications, stands apart due to its specific structure and role in research. The molecular formula—C4H5N3O2—captures everything at a glance: four carbon atoms, five hydrogens, three nitrogens, and two oxygens. Recognizing this formula unlocks the ability to dive deep into the compound’s properties and reactivity, especially if your work intersects with medicinal chemistry, microbiology, or advanced chemical synthesis.
The CAS (Chemical Abstracts Service) number acts like a fingerprint for chemicals. Every known chemical structure receives a unique number, helping researchers connect data, monitor regulations, and dodge confusion from variations in naming. For 2-methyl-4-nitroimidazole, the CAS number is 17607-44-0. This short sequence links all global data, regulatory documents, and supply chains back to the exact chemical, no matter what other names exist out there.
Laboratories, from academic to industrial, depend on exact identification. A simple mix-up can wipe out experiments, waste time, or even put health at risk. In my own experience working with chemical inventories, I have seen how a single digit off in a CAS number can end up with researchers running tests on the wrong compound. Days get lost. Forensics gets messier. Grant money gets tight. Students grow frustrated. So, when a scientist asks suppliers or regulatory agencies about 2-methyl-4-nitroimidazole, precise details save headaches and resources.
The world doesn’t move forward from textbook chemistry alone. Researchers use compounds like 2-methyl-4-nitroimidazole to push boundaries, whether they’re building new antibiotics, exploring microbial resistance, or just seeking to understand nitroimidazole as a chemical family. Interest in this specific structure often spikes when studying certain protozoal infections or investigating DNA interactions under low-oxygen conditions. For specialists researching in cancer biology or parasitology, quick and secure access to the right chemical remains key.
Mistakes happen most often with ordering and documenting chemicals. That’s rarely because of scientists skipping steps—it’s usually overwhelming databases, confusing synonym lists, or inconsistent supplier data. Making the molecular formula and CAS number a clear part of every record slashes this error rate. In my time handling chemical libraries in a pharmaceutical lab, electronic inventory systems tied to CAS numbers kept workflow smooth, audits fast, and import paperwork clean. Even regulatory compliance works best when referencing globally recognized identifiers.
Education, too, benefits when teachers put emphasis on chemical identifiers rather than relying on trade names or short forms. New chemistry students quickly develop habits for precise reporting, which helps them later in careers across research, manufacturing, and safety.
Greater industry transparency and open access to chemical databases can lighten the load for everyone—from manufacturers, to university researchers, right down to lab technicians logging samples. Keeping correct molecular formulas and CAS numbers attached to samples, research papers, and reports gives confidence and boosts collaboration, both inside and outside scientific circles. For 2-methyl-4-nitroimidazole, the details aren’t just trivia—they represent a meaningful link between solid science and safe, efficient progress.
In labs and industries, 2-Methyl-4-Nitroimidazole stands out for its strong chemical properties and potential health risks. I’ve seen far too many folks underestimate mid-tier reagents just because they’re less famous than big names like benzene or cyanide. No matter how obscure a compound sounds, its toxicity and its effects on people and the environment leave no room for shortcuts.
People sometimes settle for latex gloves, convinced they block everything. For a compound like 2-Methyl-4-Nitroimidazole, nitrile gloves give better protection. Lab coats, goggles, and a validated chemical fume hood all pull their weight. No one wants that nauseating, metallic smell lingering or, worse, to wind up coughing halfway through a routine task. From years at benches both clean and cluttered, I’ve learned spills get much more dangerous if you forget simple gear.
This stuff doesn’t belong near acids, alkalis, or anything flammable. Keep original labeling intact and store each bottle in ventilated, designated cabinets. Cross-contamination with solvents: a recipe for trouble, sometimes even explosions. More than once, I’ve seen untrained hands store incompatible substances together, thinking quick convenience trumps safety—those shortcuts don’t go unnoticed by fire marshals or insurance companies.
A little spilled powder or liquid on the floor, table, or even your gloves shouldn't set off panic but does demand a trained response. Get a chemical spill kit ready before ever opening the first container. Scoop up solids with disposable spatulas or absorb liquids with inert absorbents. Bag all cleanup materials, label that waste, and keep your workmates away from the site. No reason to improvise when simple, measured action prevents almost all accidents.
Disposal rules for chemicals in the nitroimidazole family keep changing for one reason: environmental impact. Down the drain or in regular trash—never the place for this chemical. Many towns and cities keep hazardous waste pick-up programs. If rules sound confusing, ask the local waste authority for their process. Legal disposal protects not just groundwater and wildlife, but also the people cycling that waste into the broader world. Environmental waste audits and recordkeeping—though tedious—help prevent avoidable fines and disasters.
I’ve come to value regular staff training just as much as fancy equipment. Emergency drills, clear signage, and easy-to-understand safety data sheets within arm’s reach: they shape careful attitudes. It just takes one rushed intern to crack a bottle or pour waste in the wrong container. This culture of caution isn’t about paranoia; it keeps spaces running smoothly.
Handling and disposing of 2-Methyl-4-Nitroimidazole calls for basic respect—both for the chemical’s danger and for colleagues working beside you. Common sense, regular training, and following existing waste disposal programs create a safer workplace and protect the communities around us. Nobody gets nostalgic over avoidable accidents or major clean-up bills.
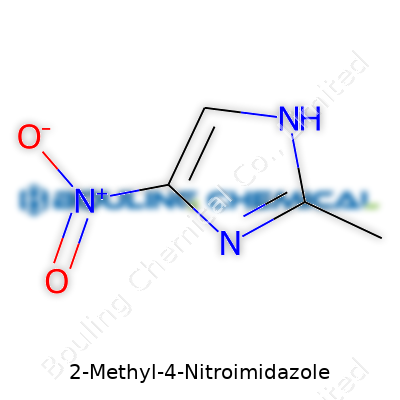

| Names | |
| Preferred IUPAC name | 4-nitro-2-methyl-1H-imidazole |
| Other names |
2-Methyl-4-nitro-1H-imidazole 4-Nitro-2-methylimidazole |
| Pronunciation | /tuː ˈmɛθ.ɪl fɔːr ˌnaɪ.trə.ɪˈmɪd.əˌzoʊl/ |
| Identifiers | |
| CAS Number | 610-21-1 |
| Beilstein Reference | 192107 |
| ChEBI | CHEBI:38722 |
| ChEMBL | CHEMBL1230103 |
| ChemSpider | 49997 |
| DrugBank | DB02908 |
| ECHA InfoCard | 03d8186f-c007-42f5-aa84-b5379f3a65e7 |
| EC Number | 259-561-8 |
| Gmelin Reference | 178405 |
| KEGG | C11238 |
| MeSH | D008879 |
| PubChem CID | 14746 |
| RTECS number | UU4025000 |
| UNII | 83CD6T6OPF |
| UN number | Not classified |
| CompTox Dashboard (EPA) | DTXSID5076788 |
| Properties | |
| Chemical formula | C4H5N3O2 |
| Molar mass | 129.11 g/mol |
| Appearance | Light yellow crystalline powder |
| Odor | Odorless |
| Density | 1.37 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 0.02 |
| Vapor pressure | 2.9E-4 mmHg at 25°C |
| Acidity (pKa) | 13.95 |
| Basicity (pKb) | 11.74 |
| Magnetic susceptibility (χ) | -32.7·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.573 |
| Viscosity | 1.1720 (mPa·s, 20°C) |
| Dipole moment | 4.62 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 117.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -67.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1884 kJ/mol |
| Pharmacology | |
| ATC code | J01XD03 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P270, P271, P301+P312, P304+P340, P312, P330, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-1 |
| Flash point | Flash point: >110°C |
| Lethal dose or concentration | LD50 oral rat 610 mg/kg |
| LD50 (median dose) | LD50 (median dose): 700 mg/kg (rat, oral) |
| PEL (Permissible) | No PEL established |
| REL (Recommended) | 0.05 ppm |
| Related compounds | |
| Related compounds |
Nitroimidazole Metronidazole Tinidazole Secnidazole Ornidazole Ronidazole |