Long before food labs started chasing the “green bell pepper” note, researchers noticed certain aromatic compounds seemed to dominate in vegetables and wines. 2-Methyl-3-Methoxy Pyrazine, known for its sharp, earthy aroma, was identified in the 1970s during the push to decode the mysteries of flavor chemistry. Early isolations traced its origins to natural crops, mainly bell peppers, where its potent character often divided opinion. Over years of analytical chemistry advancements, this compound found itself pulled out of obscurity, gaining recognition in both the food and fragrance sectors for delivering a signature grassy punch. Commercial interest surged as growers and chefs realized even a few parts per trillion could swing a dish or drink in a completely different direction.
2-Methyl-3-Methoxy Pyrazine comes as a colorless to pale yellow liquid. Its intense, instantly recognizable aroma doesn’t match its subtle appearance. Bottles often carry a strong warning about use levels, since just a drop or two can overpower mixes. What puts this compound on the map isn’t just its olfactory presence, but also its stability. This makes it especially valuable for use in perfumes, processed foods, and even tobacco products — areas where consistency from batch to batch means everything to manufacturers.
With a molecular formula of C6H8N2O, 2-Methyl-3-Methoxy Pyrazine clocks in at a molecular weight of 124.14 g/mol. Its boiling point hovers around 145°C at 23 mmHg, while the melting point drops well below room temperature, keeping it in liquid form for most applications. Its vapor is strong yet manageable; the compound dissolves easily in alcohol and ether, and resists breaking down in water at normal temperatures. Laboratory workers notice its unmistakable musty scent long before equipment picks it up, serving as a reminder of its low sensory threshold. Its logP value sits just under 1, hinting at moderate hydrophobic character—an important trait for blending in oil-rich mixtures.
Manufacturers don’t skimp on technical sheets for 2-Methyl-3-Methoxy Pyrazine. They detail purity levels (often >99%), storage recommendations (keep cool, dry, out of sunlight), and identification codes (CAS Number: 28564-83-2, FEMA Number: 3412). Labels carry hazard markers, as high concentrations may cause irritation. Nobody throws this into a product blindly; it demands careful calibration and strict batch-recording to trace any off-notes back to the right source. For shipping, the substance receives a “Caution: strong odor” advisory, reminding handlers to keep lids tight and containment lines clear.
Industrial production skips the slow grind of crop extraction, opting for efficient synthesis starting from pyrazine derivatives. Chemists build the molecule by methylating 3-methoxypyrazine under controlled conditions, usually using methyl iodide and a mild base. This route ensures purity and allows big batches to meet annual demand without seasonal swings. At small scale, labs can whip up samples for research with similar procedures, dialing in reaction times and temps to tweak the yield. The process draws on standard glassware and safety hoods because the vapor can escape fast during transfer steps.
In the field, this molecule doesn’t just sit quietly. Under the right electrochemical conditions, it can oxidize or demethylate, leading to new flavors or more subtle notes in finished foods. Adding other alkyl or methoxy groups on the pyrazine ring alters both aroma strength and volatility. Chemists sometimes modify it to reduce sensory sharpness for use in broader flavor bases, using simple hydrogenation or chlorination reactions. Each tweak gets run through both machinery and human panels, since even a minor structural change shifts the aroma profile, sometimes for the better, sometimes not.
On paperwork and global trade ledgers, 2-Methyl-3-Methoxy Pyrazine pops up under several aliases: 2-Methyl-3-methoxypyrazine, 3-Methoxy-2-methylpyrazine, and even flavor codes like “Green Pepper Pyrazine.” In fragrance catalogs, it might appear as “Bell Pepper Essence” or “Vegetal Pyrazine,” echoing its most famous aromatic property. Researchers must stay alert to these name-swaps to avoid confusion in cross-border shipments and patent searches.
Handling this compound in a lab you’ll notice strong safety emphasis—goggles, gloves, ventilated workspaces, and secure storage away from acids and open flames. Spill control plans come standard because of the intense vapor. Toxicology briefings outline that inhaling the undiluted vapors irritates airways and skin, and ingestion brings risks most food chemists prefer never to test. Manufacturing plants install multi-stage scrubbers to keep emissions under control. Regulatory bodies including EFSA and FDA place strict use limits, allowing only trace amounts in foods and fragrances to keep consumer exposure minimal.
2-Methyl-3-Methoxy Pyrazine serves the food and beverage industry well, especially in flavor creation for green vegetables, wines (notably Sauvignon Blanc), and even candies that mimic nature. Flavor houses add it to recipes to boost realism or create new taste experiences. Perfumers take advantage of its earthy note to anchor woody or forest-themed fragrances. In tobacco production, blenders use it to deepen aroma in premium blends. Synthetic biology teams now look at engineering bacteria to biosynthesize this molecule, aiming to cut costs and boost green credentials. But despite this spread, used improperly the flavor quickly turns overpowering and unwanted—a case where less always beats more.
The search for new pyrazine-related flavors drives plenty of research. Chemists probe the structure-odor relationship by adjusting substituents, hoping to unlock new taste sensations without the polarizing sharpness. Work continues on cheaper, greener synthesis using renewable feedstocks. Analysis teams run sensory trials, coupling gas chromatography with human panels to pinpoint thresholds in various matrices, from white wine to roasted nuts. Some food tech startups explore encapsulation to better control the release and longevity of the aroma. Environmental groups push hard for greener waste treatment, as production tends to generate pungent byproducts.
Studies measuring acute and chronic exposure point to low toxicity at trace use levels typical for cooking or perfumery. Still, the strong aroma marks it for extra caution: animal studies indicate some irritation at inhaled doses well above food industry norms. Researchers probe long-term effects and potential bioaccumulation, keeping an eye on allergic responses and food sensitivity issues. Regulatory thresholds always err well below any established adverse effect, thanks to the “sensory overkill” possible at less than a part per million. Ongoing surveillance continues as more industries adopt these powerful aroma chemicals.
As the demand for plant-forward flavors and sustainable fragrance solutions grows, 2-Methyl-3-Methoxy Pyrazine stands as both opportunity and challenge. Natural extraction from genetically engineered crops could reduce reliance on chemical synthesis, cutting carbon footprints across the board. Advances in micro-encapsulation may allow more precise dosing, solving the problem of over-flavoring and waste. Still, pushing into new markets like vegan foods and advanced beverages means investments in safety, communication, and labeling must keep pace. Consumer demand for transparency and multi-sensory experiences will drive companies to fine-tune both the source and delivery method for this unmistakable compound. Investing in green chemistry and smarter production offers the surest road to keeping this flavor molecule both relevant and responsibly managed in labs and commercial kitchens alike.
Open a bottle of sauvignon blanc or bite into a fresh green pepper—there’s a distinct, almost earthy aroma that jumps out. That’s often the work of 2-Methyl-3-Methoxy Pyrazine, a molecule that gives both food and wine some of their most memorable smells. It might sound like something out of a remote chemistry lab, but it’s woven deeply into what we taste and smell every day. Harvesting the right flavor in foods and beverages goes beyond picking the right crop—it’s often about managing which molecules make it to your nose and palate.
Anyone with a nose for wine knows that not every glass smells the same, even if it's the same grape. In sauvignon blanc, cabernet sauvignon, and cabernet franc, the trademark grassy and bell pepper note connects directly to pyrazines. Vineyards constantly work to balance these compounds. Too much, and wine can taste unripe or “green.” Too little, and a glass might feel flat and boring. Farmers adjust canopy management, pick dates, and fermentation styles in hopes of tuning this aroma dial just right.
Move away from the vineyard, and pyrazine’s story grows. This compound turns up in green peppers, peas, and even some beans. Food scientists add synthetic or naturally sourced 2-Methyl-3-Methoxy Pyrazine to processed foods to match or enhance expected flavors. Consider a vegan burger: by using this molecule, companies give plant-based products an earthy kick that echoes real meat or veggies. This small adjustment can hook someone who misses the aroma of a hearty meal.
Perfume makers and fragrance chemists know the punch of a few molecules. Pyrazines help put some of the “fresh cut grass” or “garden soil” vibes in candles, perfumes, and even household cleaners. These scents stir up memories or create certain moods. I remember the first time I smelled a premium candle promising a “walk through the vines”—a quick check of the label and there it was, the secret ingredient with the unpronounceable name.
Not every grower wants strong pyrazine notes. Sometimes, pests or drought can throw the chemistry of a plant off-kilter and spike pyrazine levels. Grapes suffering from sunburn or poor canopy management can taste overly vegetal. This isn’t just a taste issue. A batch of wine that’s too “green” can mean trouble for sales. Over the years, I’ve talked to growers who pick early morning to avoid the sun’s intensity or use leaf thinning to strike the right balance. Proper irrigation and pest management keep these molecules in check.
With shoppers and diners expecting bold new flavors, the pressure on food and drink companies keeps growing. Synthetic versions of 2-Methyl-3-Methoxy Pyrazine now appear in more foods and fragrances, so smarter regulation and clearer labeling help keep us honest. Farmers and winemakers chase nuance, but overdoing it can backfire. For anyone keen to learn about food, drink, or smell, understanding which molecules matter makes every bite and sip more interesting—and gives a taste of the science behind our daily ritual.
Anyone who has ever worked with or sniffed raw ingredients in a kitchen, winery, or flavor lab can tell you: certain smells plant memories. 2-Methyl-3-Methoxy Pyrazine hits the nose in a way few molecules do. It strikes quickly, introducing itself with the scent of freshly snapped green bell pepper. There’s a sharpness here, a certain clarity that cuts through even complex mixtures.
Not just green pepper—this compound reminds me of crushed green peas, or the leafy edge of a tomato vine after rain. Sometimes, there’s even an echo of earthiness, the kind of smell you get from the skin of a raw potato as you peel it. You never confuse it for anything fruity or sweet. It insists on green, and it leans toward raw.
I first picked up on 2-Methyl-3-Methoxy Pyrazine in sauvignon blanc from New Zealand, way before I knew its chemical name. That glass captured spring in a liquid—a blast of cut grass, gooseberries, and green pepper that grabbed my attention. That was pyrazine at work, bringing backbone and complexity to the wine.
Bell peppers owe much of their recognizable punch to this molecule. Even at levels that sit below taste thresholds, it shapes the experience. It’s not isolated to one table. Coffee beans, especially robusta, carry a pyrazine presence, giving that hint of vegetal or green note—sometimes adding depth, sometimes signaling an under-roasted batch.
Not every flavor journey leads somewhere pleasant. Too much 2-Methyl-3-Methoxy Pyrazine, especially in wine, can tip the balance from refreshing to jarring. It can overwhelm delicate fruit, making reds taste unripe or even harsh. If vineyard vines cool too much at night, or grapes get picked too early, winemakers risk dialing those green notes up too high.
In the kitchen, chefs have to watch out for it too. Add too many green peppers or undercooked peas to a sauce, and the taste refuses to blend in. It stubbornly stands out, reminding diners what ingredient dominated.
Winemakers and brewers face a puzzle every season. Grape ripeness, fermentation methods, even yeast selection can push the levels of this compound up or down. Some opt for blending, using more mature grapes or different varieties to tone down the green.
Chefs play with roasting and caramelizing to mellow that note, turning sharp green into something deeper and sweeter. Scientists develop new cultivars, lowering pyrazine content in peppers through selective breeding.
For those sensitive to these flavors, reading labels or asking questions in wine shops can help dodge over-the-top green aromas. For others, the aroma of 2-Methyl-3-Methoxy Pyrazine brings fond memories of gardens, fresh vegetables, honest food, and authentic wine. For me, it’s a marker: a reminder that not every flavor belongs in the background. In the right setting, this green note becomes the star.
2-Methyl-3-Methoxy Pyrazine usually pops up in stories about flavors, scents, or food research. People don’t often stop to think about what happens to these quirky chemicals once the lab day wraps up. Storage decisions can turn a prized sample into a shelf nightmare if you ignore the real quirks baked into its structure.
With this compound, stability drops fast in places where sunlight or heat sneak in. I’ve seen someone leave a capped bottle too close to a window in July—the odor changed by the time they checked again. Direct sunlight or a warm shelf changes chemical stories real quick. Pyrazines start to degrade, and the olfactory difference can mess up a month’s work if you’re handling flavor profiles or analytical standards.
Just one light bulb’s reach can ruin what makes 2-Methyl-3-Methoxy Pyrazine useful. Its aroma, often compared to earthy green peas or bell peppers, comes from tiny changes in the structure. Heat and light don’t just fade that; they turn a carefully-crafted sample into an unpredictable mess.
Lab life teaches some chemical habits the hard way. A cool, dry nook keeps this pyrazine far happier than a cluttered supply closet. In real labs, there’s rarely endless cold storage, so choices count. Chemicals like this one hold their integrity if they stay away from heat sources and humidity. I’ve tried the “freezer room is full” excuse; it never ends well for aromatic compounds. A standard practice that works involves using amber glass bottles, so light gets blocked out—no need for fancy solutions. Seal caps tightly, keep the vials upright, and check on the dates. The less you expose these bottles to air, the more true the chemical stays to its origin.
Humidity sneaks in quick wherever there are cracks or careless seals. Even desiccant packs go stale if folks forget to swap them after a while. If a compound smells stronger after a few months, something’s probably gone wrong. Too many labs keep open desiccators near a hot exhaust fan, which makes absolutely no sense if you’ve ever handled heat-sensitive aromatics. Chemical sense beats blind routine every time.
A lot of chemical storage recommendations drown in legal jargon. In day-to-day work, I’ve found blunt notes on storage jars work best—“Protect from light and heat—will lose odor!” reminds someone in the middle of a busy afternoon why the extra care matters. It’s the extra reminders, not just the SDS poster, that help everyone remember which bottle gets priority in cool, dark storage.
Storage goes beyond science for flavor scientists and perfumers. One spoiled pyrazine batch can waste weeks and drain budgets. For that reason, spreading the word about simple steps—good seals, dry spots, no light—protects both work and wallets.
Storing 2-Methyl-3-Methoxy Pyrazine isn’t just about following a checklist. It helps to respect the quirks of the molecule. A well-lit, hot shelf might suit bottled water, but it’s a disaster for delicate aromatic compounds. For something that can shift a whole fragrance or flavor batch, spending five minutes to double-check your storage saves more than just chemical purity. It saves time, money, and the real point of working with these special molecules in the first place.
Take a look at the long ingredients list on a bag of chips, a bottle of soda, or even your morning cereal. Food manufacturers love to chase certain flavors to keep our taste buds happy. One of those key players, especially in foods that need a punch of earthy, roasted aroma, is 2-Methyl-3-Methoxy Pyrazine. It shows up in everything from green bell peppers to wine. You might not know its name, but you’d likely recognize that nutty, vegetal edge in some processed foods. So, the question comes up: is it safe for us?
I dig into ingredients partly out of curiosity, but also from concern. Modern food science keeps evolving, and not every additive manages to stay in favor forever. 2-Methyl-3-Methoxy Pyrazine brings a natural appeal—after all, it occurs in veggies like peas and beans. The US Food and Drug Administration has given it the Generally Recognized As Safe (GRAS) label for use in foods, and other regions like the EU approve it within strict limits. This means, right now, food scientists and regulators agree on its safety in proper doses.
Dose matters—a lot. Even table salt or vitamin C can turn problematic if someone piles on too much. Studies on 2-Methyl-3-Methoxy Pyrazine focus on the doses actually used in food and find no concerning effects. Animal testing, which is never a fun subject, hasn’t shown any major issues when the chemical appears in such tiny amounts. Regulators do not ignore potential problems like toxicity or allergic reactions, so these approvals carry some weight.
Even with approvals in place, some folks worry about unfamiliar names in their meals. Having grown up in a family that tried avoiding mysterious food colors and flavors, I get this completely. The urge to eat food with simple, pronounceable ingredients is totally understandable. Some studies look at the long-term effects of artificial and naturally-derived flavorings. Most only test much higher levels than what ends up in food, and the evidence so far doesn’t show health risks for ordinary levels of 2-Methyl-3-Methoxy Pyrazine.
The real gray area comes from stacking lots of additives together. One flavoring at a safe dose usually stays fine, but nobody eats just one kind of processed food. Scientists often debate how these complex mixtures might affect health over the years, especially in kids. Solutions here get tricky. Clearer labeling helps, as does ongoing research. If a problem ever turns up, regular risk reassessment can catch it.
This flavor molecule’s safety record holds up so far, but people still deserve choices—and transparency. Some consumers would rather skip additives altogether and pick whole, fresh foods. Others trust that the approvals mean these chemicals are as safe as anything else in their lunch. Personal preference matters, and so does keeping up with independent food safety research. Pushing for easy-to-read ingredient lists and more honest conversations between food makers and the public helps everyone.
The short answer: 2-Methyl-3-Methoxy Pyrazine, in the small amounts found in foods, passes the current safety tests. If you’d rather go without it, more unprocessed choices fill grocery shelves than ever. At the same time, for folks who crave that earthy flavor boost, the science so far says there’s nothing special to worry about in your average snack or drink.
Every industry brings its own flavor to pyrazine usage, but the pattern stays familiar: a tiny amount makes a big difference. In the food world, especially in snack manufacturing or coffee flavoring, 2-Methyl-3-Methoxy Pyrazine typically lands in the microgram per kilogram range. This isn’t just about being frugal—it’s because the compound packs an earthy punch. One whiff and your nose registers that unmistakable green or “bell pepper” note.
Perfume makers don’t stray far from those numbers either. They mix it in at parts per million or even lower, keen to add intrigue to a scent without overwhelming everything else in the formula. In my time working with beverage developers, the same story plays out. Dosage usually clocks in below 100 micrograms per liter in spirits or craft beers—a careful hand, a steady pipette, or the batch turns unpalatable, fast.
It doesn’t take a scientist’s eye to realize pyrazines have a strong aroma. One drop in a test batch of chocolate or whiskey, and suddenly the product goes from forgettable to distinctive. This low threshold for detection keeps manufacturers on their toes. The compound isn’t expensive, but getting it wrong can mean pouring an entire vat down the drain. I’ve watched flavorists dump ruined batches after missing the mark by just a few micrograms.
Homespun winemakers and even large breweries face tough choices: push the edge and risk an overpowering note, or hold back and let the ingredient shine just enough. In the wine world, levels above two nanograms per liter often flag as faults, leading to that infamous “green pepper” character everyone debates. That’s why routine testing and titrations are staples in the lab’s playbook.
Pyrazines tend to show up naturally in foods, so regulatory bodies pay more attention to concentrations than outright bans. The FDA treats 2-Methyl-3-Methoxy Pyrazine as “Generally Recognized as Safe” up to typical flavoring use levels. The key involves staying well under 10 milligrams per kilogram for finished products.
It’s tempting to push limits in pursuit of a signature flavor, but stacking up these compounds poses risk—headaches, off-tastes, stomach complaints. That’s where experience in blending trumps theory. Set up sensory panels, test products with staff, listen to feedback. In my own stints with product launches, nothing beat that roundtable of noses and palates.
Fine-tuning dosages relies on good lab notes and a few trustworthy tools. Micro-pipettes, accurate scales, and a practice for small-scale batch testing save both money and headaches. Food scientists run trial batches, then ask for real opinions—a welcome reminder that people experience flavor differently. On the floor, blending a concentrate first, then diluting, helps with precision.
One big takeaway: don’t let protocol drift into habit. Keep records, vary test subjects, and sometimes, reset your palate with a fresh team. Adjust concentrations seasonally, since changes in raw material quality and storage can shift results. The right balance doesn’t come from charts, but from steady trial, error, and honest review.
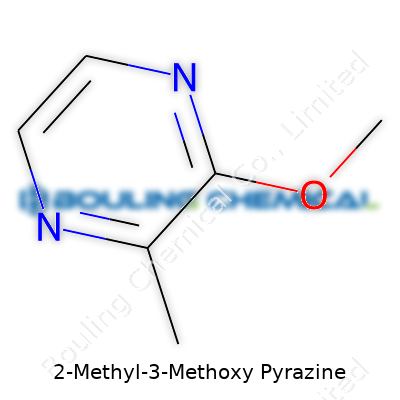
| Names | |
| Preferred IUPAC name | 2-methoxy-3-methylpyrazine |
| Other names |
2-Methyl-3-methoxypyrazine 2-Methyl-3-methoxy-1,4-diazine 3-Methoxy-2-methylpyrazine |
| Pronunciation | /tuː ˈmɛθɪl θriː ˈmɛθɒksi paɪˈreɪziːn/ |
| Identifiers | |
| CAS Number | 62511-85-1 |
| Beilstein Reference | 126786 |
| ChEBI | CHEBI:140217 |
| ChEMBL | CHEMBL190580 |
| ChemSpider | 224399 |
| DrugBank | DB11255 |
| ECHA InfoCard | 03f6c7fa-ab8c-415e-9d45-7232bfc7227c |
| EC Number | 695-91-4 |
| Gmelin Reference | 113297 |
| KEGG | C16318 |
| MeSH | D020107 |
| PubChem CID | 12015 |
| RTECS number | UG8240000 |
| UNII | D1G53K0930 |
| UN number | NA |
| Properties | |
| Chemical formula | C6H8N2O |
| Molar mass | 138.16 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Earthy, nutty, green, bell pepper |
| Density | 1.08 g/cm3 |
| Solubility in water | Insoluble |
| log P | 0.41 |
| Vapor pressure | 0.193 mmHg at 25 °C |
| Acidity (pKa) | 13.75 |
| Basicity (pKb) | 1.49 |
| Magnetic susceptibility (χ) | -72.2 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.499 |
| Viscosity | 0.862 cP (25°C) |
| Dipole moment | 2.48 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 325.67 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –19.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4276.6 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P308+P313, P321, P332+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 57°C |
| Autoignition temperature | 400 °C |
| LD50 (median dose) | LD50 (median dose): >2000 mg/kg (rat, oral) |
| NIOSH | UY8575000 |
| PEL (Permissible) | Not established |
| IDLH (Immediate danger) | No IDLH established |
| Related compounds | |
| Related compounds |
2-Methylpyrazine 2,3-Dimethylpyrazine 3-Methoxypyrazine 2-Ethyl-3-methoxypyrazine 3-Isopropoxy-2-methylpyrazine |