2-Methoxy pyrazine didn’t emerge from a singe eureka moment. Chemists in the early 20th century started isolating odd-smelling compounds in vegetables and wines. A few sharp noses and clever experiments identified this molecule’s unique profile, tracing back through grape skins and bell peppers, where it often provided that instantly recognizable earthy, almost green bell pepper note. Over the decades, food scientists figured out its pathways, while laboratories refined isolation and identification techniques. Today, I see entire teams drilling deeper, trying to pick apart how this compound shows up across plant types and fermentation steps. There’s plenty of history in how this chemical helped shape decisions in winemaking and vegetable breeding—growers adapted, sometimes intentionally lowering its levels to suit shifting consumer tastes.
Talking about 2-methoxy pyrazine feels much more like describing a quiet yet crucial ingredient that crops up in unexpected places. This compound carries a distinct aroma, much like crushed green peas or raw bell peppers, and crops up in tiny amounts in grapes, coffee, asparagus, and even potatoes. Manufacturers seek out this molecule for its punchy character, blending it into flavorings and fragrances for food, beverages, and even perfumes. Every time I’ve sampled wine or roasted peppers and noticed a strong herbal edge, I’m probably encountering this compound's signature. Commercially, it’s sold as a colorless to pale yellow liquid, carefully bottled for industrial buyers, with chemical purity ranging above 97% most times.
2-Methoxy pyrazine carries a molecular formula of C5H6N2O, giving it a modest molecular weight close to 110 g/mol. The boiling point hovers around 130–135°C at 15 mmHg, low enough that handling needs attention—fumes waft easily through a room. The compound’s density hits about 1.07 g/cm³, and its odor threshold sits astonishingly low: few parts per trillion. Telling one of my students to open a sample bottle always fills the lab with its unmistakable aroma. It mixes freely with alcohol and most common organic solvents, but barely dissolves in plain water. The chemical structure—a pyrazine ring with a single methoxy group stuck at the second carbon—means it slides into reactions expected for aromatic nitrogen heterocycles. I’ve watched it resist most moderate acids and bases, but strong oxidizers will break it down without much trouble.
On the commercial side, producers label bottles and drums with key data: chemical formula, batch number, gross and net weight, purity (usually 97% or better), and precise CAS number (with 2-methoxy pyrazine, that’s 124-16-3). Labels feature hazard symbols for irritancy, toxicity—no one wants to mistake this for a harmless flavor compound. Storage details often spell out “keep cool, dry, and away from sunlight,” because heat and light will eventually break it down. In my own workspace, tight lids and vials with solid seals are a must; even a moment of carelessness has the smell lingering for hours. Importers require safety certificates, while transport follows protocols for moderate chemical risk, especially if the shipment runs in bulk.
There are a handful of ways to prepare 2-methoxy pyrazine, and most methods draw on straightforward organic chemistry. One favored lab-scale route starts with a condensation between a substituted glyoxal and an amidine, with careful introduction of a methoxy group using methylating agents like dimethyl sulfate or methyl iodide. Reaction conditions need tight temperature and moisture control. On the industrial scale, processes lean toward using m-methoxyaniline as precursor, cyclizing under controlled pressure with ammonia or its salts. I’ve heard of routes that tweak the pyrazine ring directly, starting from methoxypyrazine carboxaldehyde. Where I work, small producers might favor older, multi-step syntheses because their reactors weren’t built for the newer processes, and it takes real foresight to avoid hazardous byproducts and batch impurities.
2-Methoxy pyrazine doesn’t just sit idly in bottles. Its reactive aromatic ring allows for further modifications—a handy trait for chemists like me. It undergoes electrophilic substitution at ring positions, swaps its methoxy group for other alkyl or aryl moieties, and reacts with a few strong bases to produce more exotic derivatives. Under ultraviolet or strong oxidative conditions, the ring can crack apart, losing its aromaticity. Labs sometimes use 2-methoxy pyrazine as a starting compound for preparing longer-chain pyrazines, which find their way into food, fragrance, and pharmaceutical industries. I’ve seen a few attempts at generating fluorinated versions for specialized sensors, though this often spirals into tedious multi-step syntheses.
Looking through catalogs and regulatory lists, I spot many names for 2-methoxy pyrazine: methyl pyrazine ether, 2-methoxy-1,4-diazine, and simply "green bell pepper pyrazine" among them. Some flavor suppliers brand it as “Pyrazine, green note” or “Methoxypyrazine (natural type)”—clearly marketing its sensory profile. CAS 124-16-3 remains the global identifier that cuts through the branding fog. I’ve found that pharmacists, food technologists, and analytical chemists may call it something different, but the chemical profile remains unchanged no matter the name.
Working with 2-methoxy pyrazine, I don’t take shortcuts. This chemical irritates eyes, skin, and respiratory tract, even in low concentrations. OSHA and EU regulations refer to it as a hazardous material. Basic handling involves gloves, eye protection, and well-ventilated hoods. Spill protocols stress quick containment and use of activated charcoal for cleanup. Storage needs locked cabinets and clear hazard signage. MSDS sheets draw attention to its moderate oral and dermal toxicity—direct ingestion or heavy skin contact triggers nervous system disruptions. Waste runs through chemical treatment rather than straight drainage, with local agencies double-checking records during periodic inspections.
2-Methoxy pyrazine holds a quiet authority in food science, particularly in flavor design. Winemakers test its levels to tweak the vegetal edge in Sauvignon Blanc and Cabernet Sauvignon, sometimes blending batches to mask or accentuate the note. Food ingredient technologists add it to savory snacks, canned vegetables, and spice mixtures to give an authentic green aroma. Perfumers sometimes lean on its earthy backbone in “green” or “fresh cut grass” scents; the smallest amounts reshape a formula. Analytical chemists sniff out trace amounts when studying environmental contamination, since its signature lingers even at parts per trillion. Every product that crosses my bench, from canned soup to herbal spirits, might offer just a trace that tips the sensory scales.
R&D projects on 2-methoxy pyrazine never lose pace. Some vineyards collaborate with plant geneticists, studying genes responsible for overproduction in grapes, hoping to find mutants that dial down the aroma without compromising yield. Analytical chemists push LC-MS and SPME-GC methods to sniff out ever-lower concentrations, supporting efforts in pesticide residue tracking and food fraud detection. Big food companies partner with academic labs, trying to mimic natural modifications that produce less off-note in peas and beans. I’ve also seen efforts in smart packaging—using scent-detecting sensors based on its molecular imprint—to catch food spoilage or packaging breaches before the eye even sees a problem.
Concerns over toxicity push researchers to dig deep. High concentrations cause adverse reactions in lab animals: central nervous system effects, respiratory distress, and mild hepatotoxicity. Long-term exposure info remains patchy—most foods never reach those levels. Regulatory bodies monitor use as a flavoring agent, capping allowances and watching for new data. Lab studies highlight irritation risks on skin and in eyes, so workplaces keep wash stations ready. I’ve followed reports from the past decade, and most point toward a manageable risk in regulated settings, but the debates over long-term, low-level exposure still trickle through research circles, especially in the context of cumulative dietary intake.
I expect 2-methoxy pyrazine’s story to keep growing. Synthetic biologists push to engineer yeast or bacteria that create the compound “naturally,” aiming for cleaner production with fewer waste streams. Plant breeders still chase new grape and pepper varieties with well-balanced flavor profiles, using genetic tools that their predecessors could only dream of. In analytical chemistry, ultra-sensitive sensors—down to a single molecule—might soon spot pyrazines with rapid, on-site devices, revolutionizing food safety and authenticity checks. With the global market for plant-based foods and sustainable farming expanding, the hunt for cost-effective, environmentally friendly, and reliable methods for producing and monitoring 2-methoxy pyrazine stands at the front of R&D pipelines, ready for the next big leap.
If you’ve eaten a bell pepper, sipped a glass of Sauvignon Blanc, or caught a whiff of a fresh garden, you’ve already come close to 2-Methoxy Pyrazine. This isn’t some laboratory curiosity; it’s a real backbone in the world of flavors and aromas. With an unmistakable earthy, green bell pepper aroma, this compound shapes how we perceive food and drink every day.
2-Methoxy Pyrazine packs a punch at incredibly low levels. Chefs talk about its effect in vegetables; winemakers debate its influence on a bottle’s bouquet. Green notes in wine spark long conversations among professionals. Sometimes Bordeaux blends or Sauvignon Blanc show these green, slightly herbal notes—the credit goes to 2-Methoxy Pyrazine. It decides if that glass tastes fresh and crisp or if, instead, it falls flat.
Over the years, I’ve seen winemakers walk the rows of vineyards in nervous anticipation, hoping for warm weather to temper these green flavors. In grapes, too much of this molecule leads to a wine that people might call “vegetal,” which folk in the industry use as a somewhat polite code for “not as ripe as it should be.” On the flip side, a touch of this compound, managed well, makes flavors lively. For example, a chef seeking to recreate the full freshness of a just-picked pea or a spicy note in a tomato sauce often has this molecule to thank.
Chemists in fragrance labs also reach for 2-Methoxy Pyrazine when they want to give perfumes an earthy, green backbone. Nature rarely provides an exact repeat, so the food and fragrance industries rely on manufactured versions to keep flavors and aromas consistent across products and seasons. If a tomato ketchup needs that little something “green” to round out its flavor, using a micro-dose of this compound can do the trick. Researchers have pinpointed its contribution to coffee, chocolate, even certain teas, focusing on how to bring out the most appealing notes in each.
Concentration matters with 2-Methoxy Pyrazine. That’s not a small detail. Too much can send flavors over the top and make a wine smell like canned vegetables or a salad bar. This isn’t just a taste issue. For grape growers, getting the balance right often comes down to harvest timing. Cooler weather and less sun mean higher levels of this molecule in grapes, so growers keep one eye on the weather and the other on the calendar.
Food safety and regulations matter, too. Any chemical that ends up in food or drink faces close scrutiny from officials and scientists. They keep tabs on how it’s used and make sure levels stay safe for consumers. The good news: decades of research support its safety in the tiny amounts used for flavoring.
Better information and more research give growers and makers the tools to control 2-Methoxy Pyrazine. Advances in molecular analysis let experts measure its levels faster and more accurately than ever before. This means fewer surprises for winemakers and a more predictable result for food manufacturers. Education has to keep pace. Emerging talent in vineyards, kitchens, and labs benefit when they understand exactly how this tiny molecule shapes a big part of our taste experience.
People may not talk about 2-Methoxy Pyrazine at the dinner table. It stands quietly behind the scenes, shaping flavors and memories all the same. In a world that asks for new tastes and reliable quality, this molecule makes its mark one green note at a time.
People might not realize how often their noses run into pyrazines. They live in bell peppers, green peas, even in a glass of Sauvignon Blanc. One small player in this aromatic crew—2-methoxy pyrazine—stands out. The chemical formula is simple: C5H6N2O. Yet even with so few atoms, the arrangement packs a punch. If you’ve ever noticed the unmistakable scent of cut grass or the snap of a green bean fresh off the vine, you’ve already met it.
Imagine a pyrazine ring—six atoms form a flat, hexagonal ring. Two opposite corners hold nitrogen atoms, while the rest are carbons. Attach a methoxy group (-OCH3) to carbon number 2; now you have the full structure. Chemists represent it like this: a six-membered ring, nitrogens at positions 1 and 4, the methoxy at position 2. Simple enough to picture, complicated enough to influence flavors in major ways.
Pyrazines don't just live in the world of test tubes. They show up in food science, perfumery, even forensic labs. The sharp green aroma that gives bell peppers their character owes much to 2-methoxy pyrazine. A few millionths of a gram can fill a room. Food engineers often chase just the right amount. Too little, flavors taste flat; too much, the food tips into bitterness or astringency.
This compound’s strength in low concentrations sometimes causes headaches for winemakers. Cool-climate grape varieties accumulate more methoxy pyrazines. Some years, a beautiful vintage risks getting labeled as ‘green’ or ‘vegetal’ because of these aromatics. The pressure to please customer tastes turns into a balancing act inside the vineyard and the cellar.
Over time, people searching for that sweet spot between flavor and off-note have tried a few things. In vineyards, leaf thinning gets more light on the fruit, which helps reduce methoxy pyrazine levels before harvest. Reducing irrigation can stress vines just enough to drop pyrazine production, too. In food plants, roasting or fermenting can transform raw ingredients, dialing down the sharpness.
Another approach involves the lab bench. Food scientists run precise analyses using gas chromatography. This machinery identifies the smallest whiff of methoxy pyrazine, helping makers control what ends up on the plate or in the glass. Winemakers lean on blending to soften harsh aromas, using wines with different levels of pyrazine. Grape breeding research focuses on developing varieties that ripen earlier or accumulate less of these aroma compounds.
Anyone who’s opened a can of peas or snapped a fresh green bean might recognize the power of these molecules. Working in a kitchen or tending a garden, the smell jumps out, full of life, sometimes overpowering. I used to think strong “green” notes meant the produce was freshest. Years in the kitchen taught me it’s easy to overdo it—sometimes a single pepper can knock a salsa out of balance. It’s funny how one small group of atoms can slice right through a complex dish.
The impact reaches beyond food and drink. Pyrazines crop up in pest control, too—some insects rely on them for chemical communication. Accidentally disrupting natural balances in crops and ecosystems because of one trace molecule isn’t as farfetched as it sounds.
With every new season, growers and makers get a better handle on the consequences of managing these molecules. Advances in analysis and breeding bring fresher produce and cleaner flavors. Understanding the chemistry behind 2-methoxy pyrazine builds stronger links between farms, factories, and dinner plates. Chasing better flavor and aroma always comes down to working with compounds at their root, not against them.
Picture sitting down with a glass of Sauvignon Blanc and picking up on that crisp, green aroma—like fresh-cut grass, maybe bell pepper. That "green" note often comes from a group of molecules called pyrazines. One in particular, 2-methoxy pyrazine, shows up in a range of foods and drinks, both naturally and because food companies add it. Think blackcurrants, green peppers, some wines, even coffee. When the flavor industry wants to give something a lift or a fresh twist, this molecule brings the taste.
Safety often boils down to how much ends up in what you eat. Natural levels in vegetables and wine haven't sparked health scares—if anything, they give character to food. Over the years, researchers have tested pyrazines on animals and cell cultures, watching for bad signs like toxicity or cancer risks. Most studies flag 2-methoxy pyrazine as safe at levels where you’d actually taste it in food. The U.S. Food and Drug Administration includes it on its list of substances generally recognized as safe (GRAS).
Europe looks at it through a similar lens. The European Food Safety Authority keeps tabs on flavoring agents and, so far, hasn’t seen red flags at levels found in reasonably consumed food. Nutrition scientists check for signs of buildup in the body, weird reactions, or allergic effects. 2-methoxy pyrazine tends to pass through the system, doing its job to add flavor, then moves along.
In a food world where synthetic and natural compounds blur together, people want to know what's in their meals. Stories pop up now and then with scary-sounding chemical names—yet most flavor molecules, even the ones invented in labs, come at such tiny doses that you'd hit a salt or sugar wall long before you could ever eat enough for harm.
I’ve been around winemakers fussing over pyrazine levels. Go too high, you get a taste nobody wants; too low, and the wine feels flat. Balance matters more. The food industry leans on scientists and regulators to set sensible limits. At the end of the day, the dose makes the difference, just like with caffeine or even water.
If you browse food ingredient lists and spot long chemical names, it makes sense to pause and wonder what's behind each one. Some folks react by pushing for "clean labels"—short ingredient lists, less chemistry on the label. But chemistry, as a science, won't go away. The key lies in research, honest reporting, and transparency.
For people worried about any flavor chemical, digging into reliable sources—FDA records, EFSA summaries, published studies—gives a better sense of where risk really sits. Personal experience tells me being curious—asking producers and searching for evidence—often brings peace of mind. Scarier-sounding compounds like 2-methoxy pyrazine invite questions, but science and history back up their safety at the amounts used in food.
In the big picture, cherishing strong food safety checks, funded research, and real dialogue with consumers keeps trust alive. Open talk about food science lets us enjoy bold new flavors, knowing someone smarter has already kicked the tires on safety.
Every winemaker or coffee roaster who spends time with their nose in a glass knows the punch of green notes that leap out in some drinks. This sharp character often points straight at a group of compounds called methoxypyrazines. Tucked among them, 2-Methoxy Pyrazine stands tall for its notorious, unforgettable impact on aroma. You know you’ve met it if you’ve ever caught a whiff of bell pepper or snapped a stem of green bean in half right next to your face — it’s that punchy sensation, part vegetable, part earthy.
2-Methoxy Pyrazine brings a scent that piles on green bell pepper, raw asparagus, and crushed pea pod, all in ways that nearly anyone on the street could call out as “planty,” even if they’ve never cracked a chemistry book. In fact, most people first experience it in Sauvignon Blanc, Cabernet Sauvignon, or even in a not-quite-ripe red pepper from the grocery store. Those same compounds give a “stemmy” edge to some wines, which can be polarizing at a tasting table. I remember the first time I stuck my nose in a glass of young Bordeaux — all I could think about was my grandma snapping beans on her porch.
The human nose picks up 2-Methoxy Pyrazine at tiny concentrations, around just 2 nanograms per liter in wine. This low threshold means its flavor has stamina — a hard worker that dominates even when it’s barely there. Wines that swing heavy on green notes sometimes get the cold shoulder from new drinkers, likely because folks expect fruit or spice and instead get hit with garden-fresh veggies. In coffee, similar rules apply. Too green or grassy and suddenly your cup isn’t comforting at all.
Growers get antsy about these compounds since ripeness plays a role in their final levels. Grapes hanging too long on the vine can let this green note fade, but pick too early and it hits like a ton of bricks. So each harvest comes with nerves — push a little for sun exposure, manage the canopy, watch the weather, all to strike a balance between vibrancy and that smothering vegetable note.
In my experience, this isn’t just inside baseball for wine professionals. I’ve gone to local tastings with folks who, after a single sniff, wrinkle their noses and declare a Sauvignon Blanc “just like eating a raw green pepper.” They’re right, even if it’s not what the winemaker hoped for this vintage. Maybe the field didn’t see enough heat, or maybe the leaves kept the grapes in shade. Either way, that green character isn’t an accident — it’s chemistry meeting climate, time, and farm decisions head-on.
Producers can’t erase 2-Methoxy Pyrazine from the process, but they have a few tricks. Site and canopy management make an impact; keeping leaves trimmed, exposing clusters to sunlight, or waiting for the perfect harvest moment lets the green fade just enough. In coffee, better roasting tempers rough and grassy flavors, which I’ve learned the hard way in my own kitchen. Someone always pours the final cup and gets to decide if that hint of green adds intrigue or ruins the batch.
This single molecule tells a bigger story about the way we engage with our senses. It draws out opinions — love or hate, vibrant or unripe. Recognizing methoxypyrazine’s role lights a bulb for anyone trying to figure out what they’re really smelling and tasting. If you’ve ever been surprised by a wine or a cup of coffee that smells less like fruit and more like your garden, there’s a good chance you’ve just shaken hands with 2-Methoxy Pyrazine.
2-Methoxy Pyrazine has a sharp, green, earthy smell. Anyone who has worked in a lab with flavor or fragrance compounds will recognize it right away. It gets used to add complexity to foods, but in its pure state, it’s powerful stuff. Safety takes real priority here, just like with any strong-smelling chemical you would not want splashed on your skin or leaking into the lab air.
Spilling a drop or leaving a bottle open can stink up an entire workspace. Once, I prepared a reference standard for a tasting panel and underestimated how persistent that scent can be. It stuck around for days, even with ventilation running. Colleagues kept asking if someone broke green bell peppers inside the lab. Simple mistakes with storage or handling spark bigger problems—both for health and the work environment.
Sticking 2-Methoxy Pyrazine on an open shelf isn’t safe, no matter how small the quantity. I keep it in tightly sealed glass bottles. I swapped out a plastic cap once and learned the hard way that vapors sneak through. Tighten with a screw cap, and go for PTFE liners if you can. Flammable liquid storage cabinets offer peace of mind, since this stuff’s flash point is on the low side. I tuck the bottle in with only similar, compatible chemicals—never close to acids, oxidizers, or food.
Light and heat beat up the molecule over time and can mess with its aroma. Best practice: cool, dry place, well away from sunlight or any heater. Think back corner of a lab fridge if you’re working with larger amounts, just label clearly and keep everything separate. Even a little spill here can taint other samples.
I don gloves before unscrewing the cap. I’ve seen colleagues get careless, only to spend hours washing off the lingering odor. Nitrile gloves offer enough barrier, and always switch out after use. Goggles shield from accidental splashes during weighing or dilution, which happens more than you think, especially when you rush. Fume hoods matter, too—draws the scent away so you don’t end up breathing it all day. Whenever I’ve taken shortcuts on ventilation, regret sets in fast. My advice: never work out in the open, even with small test portions.
Good labeling saves everyone headaches. I lost a morning searching for an unlabeled vial that someone set aside. Once it had sat uncapped, the smell alone led us to it. Hazard symbols, date received, concentration, and your initials make tracking simple. Waste containers should stand close by, so nobody walks through corridors with contaminated vials or pipettes. Small leaks or drips need absorbent pads or powder, then disposal in a sealed waste bag. A regular check of storage areas for any tacky or oily residue on bottles can stop minor problems turning major.
It only takes one incident to learn these lessons. These days, colleagues and I talk through our process out loud, double-checking one another, especially with aromatic chemicals. I push for training sessions, even if it eats into the schedule, because accidents bring real costs. Respect for these potent substances grows each time we do things right, and that approach pays off every single shift.
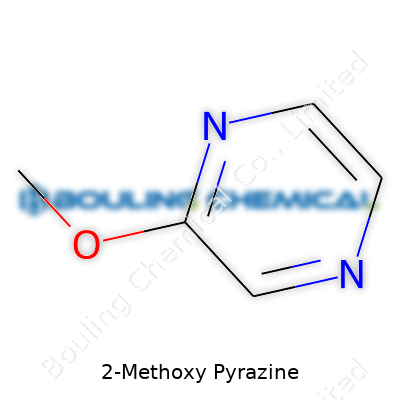
| Names | |
| Preferred IUPAC name | 2-Methoxypyrazine |
| Other names |
2-Methoxypyrazine Pyrazine, 2-methoxy- Pyrazine-2-methoxy 2-Methoxy-1,4-diazine |
| Pronunciation | /tuː ˈmɛθ.ɒk.si paɪˈreɪˌziːn/ |
| Identifiers | |
| CAS Number | 10024-66-5 |
| Beilstein Reference | 132080 |
| ChEBI | CHEBI:27533 |
| ChEMBL | CHEBI:34761 |
| ChemSpider | 122391 |
| DrugBank | DB14106 |
| ECHA InfoCard | 03eeb2b5-6125-42db-aa99-842a7a659773 |
| EC Number | 2.5.2.11 |
| Gmelin Reference | 8827 |
| KEGG | C16275 |
| MeSH | D011686 |
| PubChem CID | 13521 |
| RTECS number | UJ1050000 |
| UNII | 6Y5R8W5U76 |
| UN number | UN3350 |
| Properties | |
| Chemical formula | C5H6N2O |
| Molar mass | 124.13 g/mol |
| Appearance | Colourless to pale yellow liquid |
| Odor | Earthy, Green, Nutty, Beany |
| Density | 1.067 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.38 |
| Vapor pressure | 0.29 mmHg (at 25°C) |
| Acidity (pKa) | pKa = 2.3 |
| Basicity (pKb) | pKb = 9.51 |
| Magnetic susceptibility (χ) | 'Magnetic susceptibility (χ) = -71.0×10⁻⁶ cm³/mol' |
| Refractive index (nD) | 1.503 |
| Viscosity | 1.095 mPa·s (20 °C) |
| Dipole moment | 1.76 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 298.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -44.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1787 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06,GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: "P261, P280, P305+P351+P338, P304+P340, P312 |
| Flash point | 52°C |
| Autoignition temperature | 630°C |
| Explosive limits | Explosive limits: 1.6–12.1% (in air) |
| Lethal dose or concentration | LD50 (oral, rat): 320 mg/kg |
| LD50 (median dose) | LD50 (median dose): 40 mg/kg (oral, rat) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 2.0 ppb |
| Related compounds | |
| Related compounds |
2-Ethoxy pyrazine 2-Isopropoxy pyrazine |