Long before synthetic flavor chemists started tinkering in their labs, farmers and winemakers noticed that some vegetables, especially bell peppers, carried a pungent, earthy aroma. By the 1960s, analytical chemists pinpointed this scent to compounds called alkyl-methoxypyrazines. As flavor science grew, industry and academia took a closer look at 2-methoxy-3-sec-butylpyrazine, or MSBP. The molecule owes its early notoriety to wine industry debates, where too much MSBP made Cabernet Sauvignon taste 'green'. Over the next few decades, food chemists, perfumers, and regulatory agencies all pulled up a seat at the MSBP table, trying to understand why such a tiny molecule produced such a potent effect and how to control it in consumer products.
2-Methoxy-3-sec-butylpyrazine is a highly potent flavor compound, often described as earthy, green, and reminiscent of raw vegetables, especially bell peppers. Its detection threshold is so low—parts per trillion—that a drop in a tank can transform the entire aroma profile. MSBP appears both in natural extracts, such as those derived from green vegetables, and as a synthetic additive meant for food, beverages, and even tobacco or perfumery. In today's market, it finds wide use in flavor formulations, where recreating the "green" note of vegetables adds realism to processed foods. Cigarette manufacturers have historically added minuscule quantities to obtain a distinctive bouquet, especially for premium products.
The molecular formula C9H16N2O places MSBP among small, nitrogen-rich aromatic compounds. The structure, a pyrazine ring with methoxy and sec-butyl sidechains, means high volatility and solubility in alcohol, moderate stability in water, and a boiling point around 130-150°C. Its high volatility explains its rapid sensory impact in both food and fragrance applications. Holding a yellowish to nearly colorless appearance, MSBP can undergo slow oxidation if left exposed to air and light, which can degrade its sharpness and change the overall aroma. It’s not friendly to standard polymers used in packaging; partners need to check for compatibility to keep product integrity intact.
Suppliers offer MSBP as a neat liquid or in ethanol dilution, typically at purities of 95% or above. The compound is labeled according to CAS number 24168-70-5, with clear hazard warnings for flammability and toxicity at high concentrations. International labeling rules demand explicit mention of allergenic potential, though real-world allergies to MSBP almost never occur. Food-grade batches carry additional certification for FDA or EFSA compliance, depending on destination. Storage in cool, UV-protected glass keeps the aroma locked in, which matters for anyone seeking consistency in end products.
Synthesis in labs starts from pyrazine or precursors like 2-methoxypyrazine, followed by side-chain alkylation using sec-butyl halide under Lewis acid catalysis. This approach produces high-purity MSBP, but generates chlorinated byproducts as waste that require careful handling. Extracting the natural compound from bell peppers or peas uses solvent extraction and fractionation, but yields remain low and costs high, restricting large-scale food applications. Every lab developing MSBP must invest in scrupulous purification: trace contaminants, especially other pyrazines, can shift flavors in unwanted directions.
MSBP doesn't take kindly to rough treatment. It withstands mild acids and alkalis, but subjecting it to strong oxidizers quickly kills the aroma. Chemists interested in derivatization often swap the methoxy or sec-butyl group, aiming to tune aroma profile or volatility—the resulting compounds pop up in flavor testing panels. Hydrogenation or reduction of the pyrazine ring deactivates the odor, which provides a safety route for waste handling or accidental spills. In rare cases, MSBP gets incorporated into more complex molecules for analytical or reference standards, but the bread-and-butter use remains the parent compound.
Walk through chemical catalogs and you'll see MSBP listed under "2-methoxy-3-sec-butylpyrazine", "sec-butyl 2-methoxy pyrazine", and less commonly, "3-sec-butyl-2-methoxypyrazine". Food industry insiders call it "bell pepper pyrazine". In winemaking and brewing, it gets referred to loosely as “vegetal pyrazine” when discussing off-flavors. Official documents use the IUPAC nomenclature, but among formulators and researchers, the shorthand MSBP or simple “methoxy pyrazine” covers it. No matter the label, folks in the know recognize its punchy aroma instantly.
Handling MSBP requires gloves and proper ventilation. Although present in foods at tiny, safe levels, concentrated solutions can irritate skin, eyes, and mucous membranes. Over the years, workplace exposure studies pointed out respiratory discomfort at high vapor concentrations, which rarely occurs outside an industrial setting. Waste disposal means chemical-neutralization protocols to avoid groundwater contamination, since pyrazines can linger. Food safety regulations cap usage well below any known toxic dose, but plant managers enforce stricter internal protocols, just to keep accidental over-flavoring from ruining a production batch.
The food industry leans on MSBP to shape green profiles in canned vegetables, soups, and savory snacks. Flavored waters or “vegetable essences” tiptoe into the trend for plant-forward foods with the help of this pyrazine. Tobacco and vaping liquids, in pursuit of authenticity, throw in a trace for that characteristic leafy aroma. Perfume makers prize it in “fresh cut grass” or “herbal” fragrance blends—there’s nothing better for realism. Wine and beer folks monitor MSBP constantly, sometimes trying to minimize it for fruitier styles, sometimes boosting it for old-world character. Even animal research turns to artificial MSBP for training dogs and rodents to discriminate plant scents.
In flavor chemistry, teams race to design better ways to detect MSBP at vanishingly small concentrations. Analytical methods keep evolving, from gas chromatography-mass spectrometry to biosensors tapping into insect olfactory receptors. Breeders explore controlling MSBP in crops, seeking to tweak the aroma of vegetables and wine grapes through genetics and growing conditions. Food technologists experiment with masking and boosting MSBP, hunting the perfect balance between authenticity and palatability. Industry-sponsored research supports toxicological studies and long-term safety monitoring, especially given shifting consumer preferences for “natural” flavors; synthetic sources, despite their purity, must clear a higher bar with wary regulators.
Toxicologists put MSBP through the wringer, and results confirm it as low-risk in normal dietary exposure. High-dose ingestion in animal studies shows mild adverse effects—think reduced feeding or slight liver enzyme changes—but these appear only at unachievable levels for consumers. Inhalation studies for workplace safety reinforce the need for fume hoods in factories. Regulatory authorities worldwide assign “safe when used as intended” status, though food labeling laws require transparency. Long-term bioaccumulation isn’t a worry, given the rapid metabolic breakdown in humans and animals. Still, watchdog groups keep tabs, especially as MSBP crops up more often in new food products marketed for children.
Demand for green, fresh flavors continues to rise as more consumers chase “real” plant-based foods, leading product developers to expand their use of MSBP. Advances in biotechnology promise more sustainable production routes—engineered yeast or bacteria could make MSBP cheaper and greener than classical synthetic chemistry. Ongoing research will likely pin down more precise correlations between plant genetics and pyrazine levels, opening new avenues for crop innovation. Flavors and fragrance markets will steer the molecule into unexpected culinary and sensory territories. Safety research will keep pace, aiming to reassure customers wary of anything “artificial” in their food or drink. MSBP, with its striking potency and flexibility, looks set to influence both industrial practice and the flavors we encounter every day.
Most people have never sat down and thought about what gives green bell peppers, peas, or even certain wines that earthy, almost grassy smell. In the food world, 2-Methoxy-3-Secbutyl Pyrazine does a lot of heavy lifting. This molecule isn’t just a technical curiosity; it has earned a unique spot in kitchens, wineries, and labs. Growers, chefs, and perfumers have learned to spot its impact on both taste and aroma.
Pick up a raw green pepper in the produce aisle. That distinct, sharp scent wafting out doesn’t come by accident. 2-Methoxy-3-Secbutyl Pyrazine brings that note, even in tiny amounts. Food scientists and flavor manufacturers keep samples of it locked away for good reason. A dash here helps them bring lifelike vegetables or crisp green notes to everything from snack chips to canned soups. When frozen peas fall a little flat on the plate, formulators can bring back that lost “garden-fresh” taste with a careful touch of this compound.
Ice cream makers once relied heavily on vanilla, chocolate, and strawberry. Now consumers chase after more adventurous flavors. Add a smidge of 2-Methoxy-3-Secbutyl Pyrazine and suddenly, that corn-flavored gelato or pea sorbet stands out. The trick is getting that balance right; too much and the result tastes like you bit into a raw stem.
A serious wine taster doesn’t just swirl and sip. They hunt for complexity, looking for subtle notes hiding beneath fruit and oak. 2-Methoxy-3-Secbutyl Pyrazine brings “green pepper” and “herbaceous” flavors to red wines like Cabernet Sauvignon and Sauvignon Blanc. Growers monitor grape ripeness to steer clear of green flavors that overpower the fruit, but winemakers turn to it for character—sometimes chasing certain soil and climate combinations that nudge up pyrazine levels for leaner, fresher-tasting wines.
This can create problems, too. Weather that’s too chilly stunts grape ripening and leaves excess levels behind. Consumers start to complain about “vegetal” wine. Wineries experiment with harvest timing, leaf removal, and cellar techniques to rein things in. Getting it right creates memorable vintages. Getting it wrong means bottles sitting unsold on shelves.
Nobody wants a perfume that smells flat or artificial. Perfumers use natural-smelling building blocks like 2-Methoxy-3-Secbutyl Pyrazine to make new scents feel like a walk after a summer rain or a hand brushing through a vegetable patch. I’ve stood in a perfume lab, the air thick with test samples, and watched a chemist labor to find the right tinge of green—a process both art and science.
Many still talk about the famous perfumes from the ‘70s and ‘80s that pushed green and earthy notes. That’s where high-end creators still draw inspiration, using pyrazines to create fragrances that refuse to blend in with sugary or citrus-heavy options crowding department store shelves.
Labs use 2-Methoxy-3-Secbutyl Pyrazine as a marker for freshness and origin in vegetables. Scientists test for it in imported peas and beans, checking if storage knocked back flavor. Food quality experts count on this marker to help grocery chains deliver more flavorful produce.
In my own kitchen, noticing how a can of peas can taste different from fresh vegetables says a lot about what’s been lost during storage and transport. The more we learn about these small molecules, the easier it gets to tackle food waste and improve taste across the board.
If you’ve ever walked past a garden plot in spring or sliced open a green bell pepper, you might have stumbled into the world of pyrazines. Among them, 2-Methoxy-3-Secbutyl Pyrazine stands out for its bold, unmistakable personality. One sniff and most people frown, laugh, or wrinkle their nose. The stuff doesn’t whisper “floral” or “sweet.” Instead, it marches in with an earthy, almost aggressive punch. Think of biting into a stalk of celery pulled fresh from damp soil, or the intensity of green peas straight out of their pods. That’s the backbone of this molecule.
Describing the aroma isn’t an exercise in chemistry—experience speaks louder. Kids growing up with backyard chores know the sharp, slightly musty edge of freshly turned earth. In a kitchen, that smell rolls out whenever bell peppers hit the cutting board. It’s green, raw, strong, and some would even say a bit invasive. Food scientists peg it as one of the key notes responsible for that classic “vegetal” signature in a range of crops. In wine circles, it’s what sets certain sauvignon blancs or cabernet francs apart, stirring the debate every harvest: is it a flaw, or a mark of character?
Looking closer, this particular pyrazine isn’t content to stay in the “background notes” of flavors. In low doses, it brings crispness and life, turning a dull pepper into something vivid and memorable. In higher concentrations, though, it can dominate, overshadowing subtler fruit or floral hints. Some people appreciate that strong “green pepper” edge in wines or vegetables. Others find it overpowering, almost like the aroma of crushed leaves left under the sun. Either way, it rarely slips by unnoticed.
Pyrazines sneak into everything from vine-ripened tomatoes to salsa, coffee beans, and even chocolate. I once worked with a chef obsessed with balancing flavors in ratatouille. He saw pyrazines as the “spine” of the dish, weaving earthiness through every bite. Left unchecked, they could turn a beautiful plate into something reminiscent of canned beans or the funky undertones of an overgrown greenhouse. But with a little skill and blending, that same edge lifted the whole thing, anchoring an otherwise light, sweet profile.
This compound isn’t just locked inside gourmet kitchens or high-end wine cellars. Farmers, food manufacturers, and chefs all reckon with its presence. One reason some grapes get picked extra ripe is to let these green notes mellow out, paving the way for fruitier, rounder flavors in the final bottle. In other cases, vegetables bred for milder taste trim down the pyrazine level through hybridization, aiming to please more palates at the supermarket.
Understanding what drives these aroma molecules sheds light on the fine line between “deliciously fresh” and “unpleasantly raw.” Tossing peas into a salad, I catch that sharp green snap and remember how important timing, variety, and storage become in coaxing out the best flavors. Taste preferences drift with culture and habit—what reads as off-putting to one group turns nostalgic or even prized to another.
Farmers and winemakers meet challenges here. Too much green? Adjust harvest dates and consider training vines differently. Not enough freshness? Seek out seed varieties or fermentations that hang onto those pyrazines, letting their vibrant personality punch through. The kitchen can get creative, too—balancing with roasting, seasoning, or pairing helps tame harshness and showcase what this molecule adds. There’s no single fix, only a continual dance between nature, technique, and taste.
If you’ve ever smelled a green bell pepper or tasted a Sauvignon Blanc, you’ve probably encountered 2-Methoxy-3-Secbutyl Pyrazine without even realizing it. This chemical pops up in nature, giving certain foods their familiar earthy, sometimes grassy aroma. Food scientists use it at ultra-low levels to round out flavors in products from wine to candies.
Typical amounts in a regular diet stay low. We’re talking parts-per-billion—imagine a pinch of salt tossed into an Olympic pool. You can’t avoid this stuff if you’re eating vegetables or drinking wine. Regulatory bodies, including those in the US and Europe, have studied it, since it’s a natural part of food chemistry.
So far, there’s little evidence that the tiny traces of 2-Methoxy-3-Secbutyl Pyrazine present a health risk. Rodent studies using higher doses haven’t shown obvious toxicity or links to cancer. Still, there’s been a push for more long-term studies to address gaps. Regulators err on the cautious side, keeping an eye on new research.
For people sensitive to strong flavors or with allergies to peppers and related veggies, flavor additives in processed food can occasionally cause a reaction. That’s true of any substance, natural or synthetic, and isn’t unique to this compound.
Food makers love the powerful punch small amounts deliver. It helps produce a consistent flavor profile, especially in products like flavored beverages, baked goods, or snack mixes. Wine lovers are split; some find the aroma a prized part of a premium varietal, others think it smells like a garden after rain.
Faking freshness or making bland recipes more enticing sometimes involves spiking with nature-inspired chemicals. This isn’t so different from playing with salt or lemon zest in a home kitchen. It’s just more precise.
Tough rules cover every step from production to labeling. Major agencies—like the FDA in the US and EFSA in Europe—require detailed safety studies. These organizations cap how much can be used in food, so even if a company got creative, they couldn’t sneak in unsafe amounts.
In my own experience with food allergies, I always scan ingredient lists. Sometimes, flavor additives have long names that set off alarms. But trace amounts of 2-Methoxy-3-Secbutyl Pyrazine haven’t shown up as a problem in allergy clinics. People with rare disorders connected to pyrazines should talk to their doctor, but the average consumer faces almost no risk at the quantities used.
Following the trail of research, constant updates to food safety laws, and making label information easier to understand—it all helps build trust. More funding for independent studies would give everyone a clearer picture. Everyone from flavor scientists to food packaging designers plays a part in keeping the system safe for the long haul.
For folks who want to avoid extra flavorings altogether, whole foods and certified organic products usually keep things simple. Reading up, asking questions, and making choices based on personal needs counts for a lot.
Working with compounds like 2-Methoxy-3-Secbutyl Pyrazine always brings its own bag of responsibility. This particular chemical, showing up in everything from wine to bell peppers, packs a huge aromatic punch, and tiny traces can easily mess up a whole batch if not respected. I learned this while working in a small flavor lab. Sloppy storage meant contamination crept up at the worst times—usually when deadlines stared you down.
This molecule plays in the big leagues of aroma even at just a few parts per billion. It likes to float around and cling to surfaces or nearby samples. Give it room temperature and much oxygen, and you’ll find potency doesn’t hang around. Let’s be honest, no one enjoys dealing with evaporated or oxidized compounds, especially when the next analysis depends on purity.
The sweet spot for storing this compound: keep it cool, dry, and out of direct sunlight. My habit is to lock it away at 2 to 8 degrees Celsius, using an airtight amber glass bottle that won’t play games with UV rays. I’ll tuck these bottles in a dedicated section of the fridge, away from anything with strong odors. The material really loves to wander, so cross-contamination bites hard unless you stay vigilant.
No need to get fancy with safety, but gloves, a clean bench, and labeling with opening dates save headaches later. If someone ever leaves a cap a little loose or forgets to wipe a spill, one sniff will tell you changes happened, and not for the better. I’ve spoiled samples from lazy storage before—never want to repeat that.
This compound reacts with water, blunting its signature earthy smell. Dampness sneaks in with humidity or leaky caps. If you see any cloudiness or notice a color shift, don’t take chances—just toss it. Once, a careless friend stored a pyrazine in a sunlit cabinet, thinking the brown bottle would do the trick. The next time he checked, the aroma was all but dead.
For anyone handling this chemical on a regular basis, investing in a small, dedicated fridge pays off. Keep it away from anything else that releases odors or might spoil—your future self thanks you. Working with smaller aliquots rather than a big container means less air exposure, each time you need some. This habit has saved my samples more times than I can count.
Proper storage sounds simple but slips through the cracks when you’re busy. Trust me, one lazy moment brings ruined batches and wasted work. Whether in the food and beverage world or a research setting, the calmer you keep 2-Methoxy-3-Secbutyl Pyrazine, the truer its aromas stay, and the less you need to worry about accidental surprises.
Ask anyone familiar with flavor or fragrance formulations and they’ll nod in recognition at the sharp, green character 2-Methoxy-3-Secbutyl Pyrazine brings to a blend. It’s not just some mystery molecule made for chemical databases. In practical terms, its purity or concentration shapes everything from its performance in flavor mixes to how strict the regulatory bodies get on its usage.
The industry doesn’t leave much to guesswork where purity matters. Commercial sources of 2-Methoxy-3-Secbutyl Pyrazine often provide purity levels of 95% or above. Chemists and buyers scan technical data sheets for those numbers, hunting for batches in the 98–99% purity range. The margin matters a lot because impurities play spoilsport—off-notes, unwanted flavors, even headaches over safety compliance.
A handful of years working alongside flavorists taught me that higher purity isn’t just about prestige. It’s about control. In flavor testing, a 95% pure sample versus a 99% pure one can turn a winning formula into something barely palatable. Lower purity sometimes means lingering unpleasant aromas or less predictable outcomes batch after batch.
Lab teams invest heavily in refining synthesis because food safety agencies, especially in the EU and US, put strict rules on allowable impurities, sometimes as low as 0.1%. In regulatory documentation and food safety submissions, buyers look for Certificates of Analysis proving these tight specs are reached. If a company spots a lot consistently stuck at 93%, those drums collect dust. The difference between 98% and 99% can tip the scales between a product getting green-lit for release or getting held back for more cleaning up.
On the other end, extremely pure samples above 99% aren’t always necessary and can rack up steep costs with little added value. Chemical separation and purification demand energy, solvents, labor—all things that jack up the price per gram. You start seeing diminishing returns, especially where tiny amounts do the job just fine.
Food companies use 2-Methoxy-3-Secbutyl Pyrazine at very low concentrations—parts per billion or even lower. Too much, and things run wild, with a bell-pepper intensity that throws everything off balance. Manufacturers gravitate toward the most reliable and pure variants, which mix in clean, without throwing batches out of spec. From my experience in trial runs, lower purity sources often force panelists to reset more often because of lingering aftertastes, slowing product development and hurting trust in new suppliers.
The hurdles start with raw material sourcing. Crop failures or delays in precursor chemicals can push prices up and leave bottlenecks in supply. Not every supplier meets those tight purity levels. Some slip up and deliver inconsistent quality, leading to headaches for manufacturers reliant on steady, reliable sources.
One solution comes from supplier diversification. Teams I’ve worked with vet and onboard multiple suppliers, building relationships in different regions and maintaining strong QC through third-party labs. Others invest in on-site purification, adding a safety net when incoming batches fall short of spec. More dialogue between producers, buyers, and regulators helps smooth wrinkles before they turn into costly recalls or formula rework.
At the end of the day, purity becomes more than a number on a data sheet—it's a foundational piece in safe, flavorful, and trustworthy products.
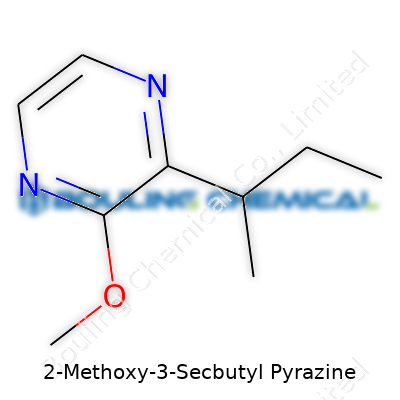
| Names | |
| Preferred IUPAC name | 3-(Butan-2-yl)-2-methoxypyrazine |
| Other names |
2-Methoxy-3-(1-methylpropyl)pyrazine 3-Isobutyl-2-methoxypyrazine SBMP |
| Pronunciation | /tuː-mɛθˈɒksi-θriː-sɛk-ˈbjuːtɪl paɪˈreɪziːn/ |
| Identifiers | |
| CAS Number | [24168-70-5] |
| Beilstein Reference | 634172 |
| ChEBI | CHEBI:132670 |
| ChEMBL | CHEMBL2106699 |
| ChemSpider | 21541097 |
| DrugBank | DB03446 |
| ECHA InfoCard | 20-201-280-6 |
| EC Number | 425-860-5 |
| Gmelin Reference | 607861 |
| KEGG | C18367 |
| MeSH | D026587 |
| PubChem CID | 61353 |
| RTECS number | UJ6475000 |
| UNII | FF7048S7A5 |
| UN number | UN3352 |
| Properties | |
| Chemical formula | C9H16N2O |
| Molar mass | 166.24 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Powerful, green, earthy, bell pepper, galbanum |
| Density | 0.979 g/mL at 25 °C |
| Solubility in water | Insoluble |
| log P | 1.80 |
| Vapor pressure | 2.16E-02 mmHg at 25°C |
| Acidity (pKa) | 13.38 |
| Basicity (pKb) | 1.2 |
| Magnetic susceptibility (χ) | -76.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4610 |
| Viscosity | Liquid |
| Dipole moment | 2.15 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 395.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -187.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4990 kJ/mol |
| Pharmacology | |
| ATC code | N06DX52 |
| Hazards | |
| Main hazards | H315, H319, H335 |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P273, P280, P305+P351+P338, P337+P313 |
| Flash point | 65 °C |
| LD50 (median dose) | LD50 (median dose): **>2000 mg/kg (oral, rat)** |
| NIOSH | MW3850000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 ppb |
| Related compounds | |
| Related compounds |
2-Isobutyl-3-methoxypyrazine 2-Methoxy-3-isopropylpyrazine 2-Methoxy-3-ethylpyrazine 2-Methoxy-3-sec-pentylpyrazine 2-Methoxy-3-(1-methylpropyl)pyrazine |