Curiosity about pyrazines goes back to the golden age of organic chemistry. The trail of 2-Methoxy-3-Isopropyl Pyrazine (MIP) leads through decades of aroma research, with roots anchored in wine cellars and vegetable gardens. Years ago, the pungent scents of bell peppers and certain Sauvignon Blancs drove scientists to figure out what made them so distinctive. Tucked inside these sharp aromas rests MIP, only detected once analytical tools outgrew crude distillation and entered the territory of gas chromatography and mass spectrometry. With progress in extraction and identification, MIP turned from an obscure plant metabolite into an industry molecule that chemists could produce in a lab. Today, the flavor and fragrance world sees MIP not just as a quirky plant compound, but as a force steering sensory qualities in food, beverages, and even pest control.
2-Methoxy-3-Isopropyl Pyrazine catches the attention of those looking to punch up or play down “green” aromas, since it crystallizes that signature, earthy bell pepper character. For anyone mixing flavors or replicating natural scents, MIP acts as a reliable molecular dial—just a trace can shift a beverage or perfume from flat to intriguingly vegetal. Producers package it in high-purity, liquid, or crystalline form for use in flavor, fragrance, and even crop protection. Strict labeling and tight control over concentrations soften its impact, because this molecule leaves a strong mark even at astonishingly low levels, measured in parts per trillion in food context.
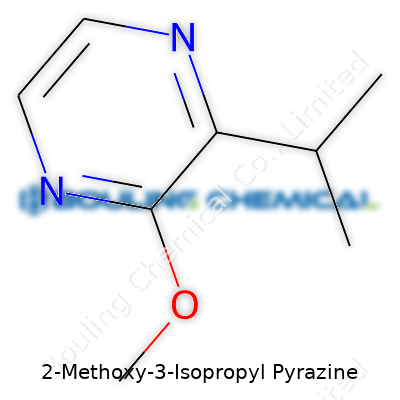
One sniff of pure 2-Methoxy-3-Isopropyl Pyrazine, and the nose wakes right up—it’s a colorless, sometimes pale yellow liquid with a powerful green, earthy odor. It shows a knack for volatility, drifting into the air at room temperature and carrying its scent far and wide. On a molecular scale, it carries the typical pyrazine structure: a six-membered heterocycle with nitrogen at the 1 and 4 positions, an isopropyl peg at the 3 position, and a methoxy handle at the 2 position. With low water solubility and moderate solubility in organic solvents, it often shows up in ethanol, ether, and dichloromethane extractions. The boiling point lands just shy of 200°C, and its stability holds up across moderate temperatures, though sunlight and strong acids can eventually break it down.
Producers make it a point to hit 98% or greater purity, verified by high-performance liquid chromatography or gas chromatography. Labeling must line up with international transportation laws, since this compound’s odor can trigger headaches and nausea at high exposures. Safety data sheets emphasize not just the chemical makeup but emergency handling, environmental hazards, and cradle-to-grave storage practices. Food-grade or fragrance-grade labels set expectations about potential traces of solvents and the strict absence of other pyrazine isomers, since even small impurities can nudge the scent or flavor profile off course. Lot numbers play a role in traceability, tracking every batch from lab to loading dock.
Lab synthesis relies on two primary paths. The first starts with 2-methoxypyrazine and brings in isopropyl groups through targeted alkylation, often with isopropyl bromide and base at cool temperatures to limit side reactions. The alternative runs a condensation between methyl glyoxal, isopropylamine, and methoxy components, followed by ring closure and purification. Extracting MIP from natural sources, while possible, takes pounds of vegetal material for even a sniff of pure compound, which pushes most manufacturers toward total synthesis. Post-reaction, chemists run a series of distillations and crystallizations, keeping eyes peeled for isomeric impurities or lingering base residues that can dull the final aroma.
The methoxy group at the 2-position allows for selective demethylation, sometimes producing 2-hydroxy derivatives with altered aromas. Nitration rarely touches the pyrazine ring, but more forceful oxidizers or acid chlorides will tear it apart. Reduction with strong agents targets the nitrogen in the ring, which can shut down its signature scent entirely. Skilled chemists tweak the side chains, swapping out isopropyl for tert-butyl or sec-butyl, hunting for even sharper or softer aromas. These molecular games play out not just in flavor labs but in agricultural studies, where modifications tune persistence on crops or in the environment.
Step into a flavor catalog, and you’ll find 2-Methoxy-3-Isopropyl Pyrazine under names like “IPMP,” “Isopropyl Methoxypyrazine,” or “Bell Pepper Pyrazine.” Suppliers sometimes abbreviate it as “MIP” or drop in flavor-speak like “green pyrazine.” In chemical indexes, accuracy rules, and the IUPAC name—2-methoxy-3-(propan-2-yl)pyrazine—sits beside more user-friendly tags. Market names play with the sensory pull, using descriptors like “vegetal essence” or “green note enhancer” to reach chefs and perfumers who may not speak organic chemistry.
Direct exposure to concentrated MIP can irritate eyes and airways, sometimes causing headaches or dizziness, so handling sticks to gloves, fume hoods, and sealed vials. In the factory or lab, storage stays below 25°C, away from light and out of reach of oxidizers. Spills get covered with inert absorbents—no splashing water or strong acids, which may spread the odor or trigger decomposition. Fire fighters need to know that, while the compound itself isn’t wildly flammable, its vapors can ignite in confined spaces. Standards from REACH, OSHA, and local food authorities guide the path from raw chemical to finished flavor, highlighting maximum residue limits and strict separation between work and food areas. Anyone working near it learns fast—trace amounts stick to clothes, and even a diluted solution shouts its presence across a full building.
In winemaking, the presence of MIP can spark heated debates. Too much can push a wine past the edge, overwhelming fruit notes and sinking a vintage to the bottom shelf. Get it right, and it sharpens complexity, sending taste buds chasing after subtle green sparks. Pepper growers, beer brewers, and even some chocolate makers wrestle with the same balance, tuning crop species and process steps to dial MIP up or down. Beyond food, pest control research finds MIP helpful as a mimic of natural warning signals between plants and insects, manipulating bug behavior in orchards and fields. Some perfumers chase it for garden-fresh colognes, eager to catch the fleeting promise of a newly cut lawn or fresh snap pea. What stands out is its power—trillionths of a gram change the game and draw both admiration and caution in equal measure.
Recent years see research racing into both detection and control of MIP, especially as climate shifts and crop varieties throw flavor profiles into chaos. Analytical labs turn to ever-faster, higher-sensitivity tools, refining both static and dynamic headspace analysis to pick out MIP’s ghostlike signature. In the laboratory, the hunt for more sustainable synthetic methods picks up steam—whether through enzyme catalysis, greener solvents, or biotransformation. Collaborative studies span continents, with grape growers, bean processors, and chemists sharing notes to outmaneuver both too much and too little of this compound. Even small miss-steps ripple out, affecting not just one bottle of wine or batch of bell peppers, but regional export values and consumer reputations.
Toxicologists track MIP exposures down to the smallest levels, since this compound carries a stubborn tendency to be noticed by human noses long before it reaches dangerous concentrations. Animal tests flag a risk only at levels rarely approached outside of industrial accidents, with most food exposures sitting thousands of times lower. Long-term research focuses on any tie to allergies or chronic irritation, asking whether continued exposure—say, in a bottling plant—leads to subtle health effects. There’s also an environmental angle, as traces in wastewater could build up with heavy synthetic use. Regulators monitor not just human impact, but what happens downstream to insects, beneficial soil bacteria, and aquatic life if concentrations climb.
Growing demands for nuanced, plant-derived flavors put MIP in a unique position. As consumers swing toward natural foods and authentic aromas, both synthetic and natural-extracted MIP see strong demand in global markets. Tech-driven agriculture looks for ways to block excess MIP in grapevines bred to higher yields, while urban farming circles ponder genetically tweaking aroma pathways entirely. In regulatory limelight, traceability and food safety audits become more rigorous, prodding producers into greater transparency on sources and processes. As more industries learn to manage both the promise and pitfalls, MIP’s story keeps evolving—bridging the world between sharp-eyed chemists, restless growers, and everyday taste explorers.
2-Methoxy-3-Isopropyl Pyrazine flies under the radar for most people. The name sounds like something out of a chemistry quiz, yet it’s about as tied to everyday life as coffee or a glass of Bordeaux. Just a whiff triggers memory—green bell peppers, earthy wine, even the crunchy sweetness of some snack foods. This molecule carries a punch of aroma and taste, and that’s exactly why it gets so much attention from food scientists and winemakers.
Take a sip of Sauvignon Blanc, and the green, vegetal notes aren’t by accident. 2-Methoxy-3-Isopropyl Pyrazine builds the backbone of those grassy and peppery layers. For many wine lovers, too much can tip a bottle toward harshness, but in the right amount, it turns simple juice into something complex. Old World Bordeaux and New World Cabernet blends owe a bit of their style to this molecule.
Then think about food—not just the fresh crunch of bell peppers, but certain peas and asparagus. Chefs talk about “green” flavors. Those flavors don’t just come out of nowhere. Their intensity often boils down to pyrazines. Some snack manufacturers even lean into this, using lab-made versions to lock in a consistent flavor profile that never lets down, batch after batch. The food industry doesn’t just accept pyrazines—they seek them out and dose with care.
2-Methoxy-3-Isopropyl Pyrazine proves a point: a trace of the right compound changes a meal or a glass of wine. Back in college, I wondered why some veggies tasted more “green” than others, even when cooked the same way. Later on, I learned how much came down to these molecules, which can be detected by the human nose in a part-per-trillion range. That’s powerful stuff for something too tiny to see.
For growers and vintners, it’s a double-edged sword. Too much pyrazine brings sharpness nobody signed up for, and weather plays a role—cooler growing seasons often put more of it into grapes and, eventually, wine. In the field, farmers choose how long to let crops ripen to keep the green notes in check. Winemakers might adjust fermentation tricks, blending, or even try fining agents to pull those flavors back a notch.
Aromatherapy fans sometimes point to naturally-sourced molecules for their freshness, trying to capture the “garden after rain” smell for candles and sprays. 2-Methoxy-3-Isopropyl Pyrazine turns up in some fragrance blends, especially those with green and herbal top notes. The same goes for specialty coffee folks analyzing beans for “off” flavors that come from unripe coffee cherries. Controlling pyrazine content gets serious attention, as it spells the difference between a world-class batch and one that sits unsold.
Researchers keep looking for ways to measure this molecule more precisely. DNA studies and advanced analytics could help breeders develop grape or vegetable varieties that hit the tasting sweet spot. The careful use of 2-Methoxy-3-Isopropyl Pyrazine shapes not only what we eat and drink, but how we experience freshness and flavor. If folks pay attention, this powerful chemical could turn everyday food and drink into something memorable—no chemistry degree needed.
Give 2-Methoxy-3-Isopropyl Pyrazine a sniff, and subtlety leaves the room. This molecule’s aroma stands out as one of the most potent and unmistakable. It reminds me of harvesting green bell peppers straight from a sun-warmed garden—just crush a leaf or nip a raw pod and sense that zesty, earthy bite hit your nose. No pretending: there’s nothing soft or ambiguous here. Most people will pick up the green, vegetal sharpness right away, along with a certain musty note like freshly snapped pea pods or just-unearthed potatoes. Winemakers and distillers call it “green pepper,” brewers sometimes grimace and say “raw pea.” Either way, this chemical pulls up a seat at any table and makes itself known.
2-Methoxy-3-Isopropyl Pyrazine doesn’t just appear in labs. It’s floating in everyday foods and drinks, so strong that just a trace can infuse a batch of wine or a handful of vegetables. You’ll find the telltale aroma in bell peppers, peas, and even some types of Sauvignon Blanc and Cabernet Sauvignon grapes. I remember swirling a glass of young Cabernet and catching that “green” edge before any fruit notes—winemakers both dread and admire it. In some climates, grapes gather more of this compound, giving rise to heated debates about whether “herbaceous” flavors help or hinder a vintage.
Barely a few parts per trillion can trigger a sensory response. This means winemakers and brewers spend a lot of energy tracking levels, knowing that just a whisper too much turns a bottle from “vivid” to “vegetal.” It’s easy to undervalue such a tiny presence, but anybody who’s had unpleasantly green-tasting asparagus in early spring, or a pale ale with a weird edge, knows a little goes a long way. The same quality that brings fresh vitality to certain wines or peppers can also overwhelm, sparking reactions from admiration to outright rejection. I’ve seen tasting groups argue for hours over whether that snap of green means pedigree or mistake.
Fragrance brands chase this molecule too. Seek out a perfume that wants to mimic the wild woods after rain or the stem of a tomato plant, and you’ll probably find a drop of 2-Methoxy-3-Isopropyl Pyrazine hiding somewhere in the formula. It taps into memories for people—muddy hands from a garden, or the fresh-cut grass of a soccer field after a storm. Scent can stir up nostalgia, and this compound packs enough personality to summon a whole season or place.
Managing this molecule’s strong notes presents a puzzle. There’s a fine line between lively and overbearing, especially in wine or food that’s meant to be delicate. Farmers adjust harvest times, grape growers tweak sun exposure, brewers test roast profiles. Fermentation and aging processes can tame or exaggerate the sensation, meaning trial and error rules the day. No easy solutions—only experience, careful timing, and respect for nature’s own chemistry set. For me, few things beat that moment a chef or winemaker finds the green just right—when a touch of this scent brings the whole story together.
2-Methoxy-3-Isopropyl Pyrazine brings an unmistakable earthy, green pepper aroma—anyone who works in food science, flavors, or even fine wine circles has run into that sharp scent. Behind the notes and flavor chemistry, this compound requires mindful storage and safe handling, not just to protect its character, but also to keep those using it out of harm’s way. One time while dabbling in sensory science, I learned quickly how even a few drops can overtake a whole lab if you don’t treat it right.
You can usually spot the right spot for a chemical by asking yourself if you’d leave your favorite chocolate bar there—dark, dry, and cool does the trick. 2-Methoxy-3-Isopropyl Pyrazine lasts a lot longer and stays true to form when stored in a place like that. Stashing it on a sunny windowsill or near a heat vent? That’s just asking for trouble. Light and warmth push many organic compounds to break down or get more reactive. In this case, you don’t want a change in odor profile, or a risk of the compound becoming unusable.
Seal matters just as much. Once, I saw someone leave a bottle cracked open for “just a second” so they could come back and measure more later. The whole corner of the storage area smelled like green bell peppers for two days. Air and moisture creep in fast, speeding up breakdown. Screw caps down tight. Eliminate excuses.
Contamination is a big deal; the tiniest spill means lasting scent in a place you may not want it. Glass works best for storage, since some plastics absorb odors or even leach small chemicals if left too long. Personally, I always write the date received and first opening directly on glass bottles. Freshness counts, and old samples get strange after a year or so.
Opening pyrazine in a cramped or poorly ventilated room feels like inviting a pepper factory into your workspace. If you've ever been in a room after someone’s been overzealous with this stuff, you know the scent lingers for days. Out in the open, or under a proper fume hood, you won’t have to explain to your coworkers why everything suddenly smells “off.”
Lab coats and gloves aren’t just for accident-prone folks. Pyrazines stick to skin and clothing, much like how garlic lingers after chopping. Think about your own experiences in kitchens or labs—stains and smells stick around until you scrub, and sometimes even then. Eye protection helps, too. You can recover from odd seasoning in dinner, but it’s not so easy to undo chemical exposure.
Spills and splashes sneak up. Always keep some absorbent material on hand—paper towels soaked in warm water, plus a way to seal up contaminated waste. Disposing of waste makes a difference. Local rules usually say how to handle aromatic chemical waste; follow them or risk a surprise next time you empty the garbage. Ventilate, confine, and don’t let housekeeping slide.
Anyone who deals with aromatic compounds knows freshness, precision, and respect make daily handling safer. Masking off storage, labeling everything, and having a solid cleanup plan add up to a workspace where accidents stay rare and the original goal—quality science or flavor—remains possible. I’ve found that even if you only handle a vial once a year, the small habits always pay off in peace of mind.
Open a bottle of Sauvignon Blanc or eat a handful of green bell peppers, and you’ll pick up a certain earthy, almost grassy hit. That scent comes from a family of chemicals called pyrazines, and among them, 2-Methoxy-3-Isopropyl Pyrazine stands out for its punchy, unmistakable flavor. This molecule has found its way into foods and drinks, especially where people want to mimic or boost “green” and “vegetal” notes.
Chefs and food manufacturers love building powerful taste experiences. A dash of 2-Methoxy-3-Isopropyl Pyrazine can intensify the experience of certain fruits, vegetables, coffee, and even wine. Its strength lies in its ability to deliver a big sensory impact even at minuscule levels. The detection threshold for this compound can drop as low as a few parts per trillion—so it doesn’t take much to change a whole batch of food or drink.
Regulators and scientists have paid attention to this compound, mostly because it’s naturally present in foods and, in pure form, gets used as a flavoring. Groups like the US Flavor and Extract Manufacturers Association (FEMA) have looked at safety, considering the small amounts used. They gave it the all-clear as a flavoring substance, echoing similar decisions in Europe. They check toxicology data, structure, how the body breaks it down, and documented effects at typical levels.
In my own experience tasting new foods, the tiny amounts added for flavor barely compare with what you’d get from, say, roasted coffee beans or certain raw vegetables. This matters because understanding exposure helps keep the safety discussion grounded—eating a normal diet already gets you low doses of pyrazines straight from nature.
It’s tough ignoring the fact that folks have grown uneasy about “chemicals” in their food. With strange names and synthetic origins, compounds like 2-Methoxy-3-Isopropyl Pyrazine draw suspicion fast. I’ve watched people snub their noses at ingredient labels that sound like a science experiment. The real trick is remembering how many of these molecules come from the foods we already eat. Cooks and home bakers use extracts, yeasts, and all sorts of concentrates to create flavors—no one bats an eyelash at vanilla extract, but mention an unfamiliar molecule and worry spreads.
Science helps by showing that the amounts used in food production don’t cause harm when eating a mixed, real-world diet. The body can handle these tiny traces without trouble. What raises red flags—no matter the chemical—is when manufacturers push limits for cost reasons, or skip safety checks. Oversight and rules keep these risks in check, but vigilance matters.
People want honest information. Food makers sometimes hide behind jargon, and that helps nobody. If you tell diners, “This flavor is also in bell peppers and coffee,” the anxiety drops. Being transparent, sourcing responsibly, and sticking with tested uses go farther than any marketing spin.
Anyone worried about synthetic additives can look for ingredient lists and stick with whole foods. Regulators can keep pressing for research, so that even obscure compounds don’t slip under the radar. Real food safety comes from a mix of sensible rules, honest science, and clear communication—not scaring folks with strange-sounding names.
Anyone who's ever cracked open a bell pepper or sniffed the skin of a Sauvignon Blanc grape has probably crossed paths with 2-Methoxy-3-Isopropyl Pyrazine (MIP). There’s no denying how a pinch of this molecule can fill a space with that vivid, unmistakable green note—part garden, part fresh-sliced produce. But how much of it typically shows up in real-world applications? That’s where the story gets interesting, and a little goes a long way.
From years of talking to perfumers, food technologists, and enologists, one thing stands clear: MIP is potent. In most fragrance or flavor work, concentrations rarely push past one part per billion (ppb). It can seem astonishing, but at levels as low as 1–20 nanograms per liter (ng/L), the presence already leaps out. In the world of food flavors, you might spot it near 0.1–2 ppb, especially in vegetable-forward profiles, where going heavy-handed turns 'fresh' into 'overpowering' in a flash.
Winemakers, on the other hand, spend a lot of time measuring and managing MIP. In Sauvignon Blanc, numbers often drift between 5 and 20 ng/L. At much above 30 ng/L, most tasters start to notice that signature bell pepper edge, which some folks love and others run from. My time with grape growers in New Zealand showed that harvest timing and canopy management influence this number just as much as production choices down the line. A late harvest brings the MIP down, but at the cost of the zing some winemakers seek to preserve.
In the flavor industry, product developers chase authenticity. Green pea, asparagus, tomato, and capsicum notes need just a drop of MIP to catch attention. Synthetic samples come ultra-diluted, usually around 100 ppm in a carrier, to allow precise dosing. In finished retail food products—think vegetable soup or green salsa—the finished level seldom tips over a few tenths of a ppb, since most palates recoil at anything higher.
A lot of my friends in perfumery have this molecule stashed for that sharp, instantly-recognizable “green twist.” They warn about dosing under the threshold to avoid a clash with floral notes. In fresh, aromatic perfumes, usage levels barely crest 0.001% of the fragrance oil.
People can pick out MIP at remarkably low levels—some reports peg human perception thresholds below 2 ng/L in water and even less in alcohol. In beverages, especially, just a fraction too much spoils the entire batch. Labs keep updated with high-sensitivity detection gear for this very reason. Mistakes don’t slip past consumers; too much MIP quickly gets labeled as “vegetal off-note” and products find themselves left on shelves.
Instead of going higher, product makers control the harvest, source, or even try natural fermentation tweaks to reduce MIP before it ever becomes an issue. Some vineyards have shifted toward lighter canopy exposure to moderate the compound in grapes. In flavor work, blends lean on other green notes less pungent than MIP, using it as an accent rather than a headline.
Precision in measurement and a good nose are only part of the answer. Improved crop management, advances in fermentation science, and better blending skills all help wrangle this powerful molecule. In my own experience, you appreciate the punch of MIP best in modest amounts, where it teases the senses without bludgeoning them. So, while the chemical can power up the profile of a food or drink, moderation, creative sourcing, and accurate testing keep those bold flavors tasting just right.
| Names | |
| Preferred IUPAC name | 3-Isopropyl-2-methoxypyrazine |
| Other names |
O-Methylisopropylpyrazine 2-Methoxy-3-(1-methylethyl)pyrazine 2-Methoxy-3-isopropylpyrazine 2-Isopropyl-3-methoxypyrazine 3-Isopropyl-2-methoxypyrazine |
| Pronunciation | /tuː ˈmɛθ.ɒk.si θriː aɪ.səˈproʊ.pɪl paɪˈræziːn/ |
| Identifiers | |
| CAS Number | 24683-00-9 |
| 3D model (JSmol) | `3D JSmol string` for **2-Methoxy-3-Isopropyl Pyrazine**: ``` methoxy n1c(ccn=c1C(C)C)OC ``` This is the SMILES string: `COc1nc(C(C)C)ccn1` You can use this SMILES string in JSmol or any compatible 3D molecular viewer. |
| Beilstein Reference | 107873 |
| ChEBI | CHEBI:141499 |
| ChEMBL | CHEMBL3182215 |
| ChemSpider | 224468 |
| DrugBank | DB14162 |
| ECHA InfoCard | 03e553f5-d47b-4b5c-90de-d9c8263897e3 |
| EC Number | 26024-36-0 |
| Gmelin Reference | 82742 |
| KEGG | C14389 |
| MeSH | D020115 |
| PubChem CID | 122247 |
| RTECS number | UD2300000 |
| UNII | PEY4T49S9K |
| UN number | UN3334 |
| CompTox Dashboard (EPA) | DTXSID5020719 |
| Properties | |
| Chemical formula | C8H12N2O |
| Molar mass | 180.24 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Earthy, green, bell pepper |
| Density | 1.018 g/cm³ |
| Solubility in water | Insoluble |
| log P | 0.83 |
| Vapor pressure | 0.0155 mmHg at 25 °C |
| Acidity (pKa) | 12.3 |
| Basicity (pKb) | 10.71 |
| Magnetic susceptibility (χ) | -74.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.498 |
| Viscosity | Viscous liquid |
| Dipole moment | 1.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 332.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4552.7 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P362+P364, P501 |
| Flash point | 68°C |
| LD50 (median dose) | LD50 (oral, rat) > 5000 mg/kg |
| NIOSH | UH8225000 |
| PEL (Permissible) | Not Established |
| REL (Recommended) | 0.002 ppm |
| Related compounds | |
| Related compounds |
2-Methoxy-3-sec-butylpyrazine 2-Methoxy-3-isobutylpyrazine 2-Methoxy-3-ethylpyrazine 2-Methoxy-3-methylpyrazine 2-Methoxy-3,5-dimethylpyrazine |