Long before labs filled with today’s high-tech equipment, grape growers and winemakers noticed how some wines carried a punchy, unmistakable green, vegetable-like scent. By the 1960s, chemists tracked it to an elusive group: the alkyl-methoxypyrazines. In the decades that followed, the structure and characteristics of 2-Methoxy-3-Isobutyl Pyrazine (MIBP) emerged from the haze. Isolating the molecule required a lot of trial and error in distillation and extraction. By the late 1970s, researchers grew to appreciate its role not only in grapes, but in everything from bell peppers to hops, and even in certain animal secretions. With this new knowledge, food technology shifted, passing from the hands of scientists into the realm of industry, where “green notes” suddenly became a tool rather than a flaw.
Walking into any facility that blends fragrance for food and beverages, you might spot a small amber bottle labeled “MIBP” or “Green Bell Pepper Extract.” By now, companies source and manufacture this compound for use across multiple markets. In wine, a few drops can amplify or mask specific notes. Perfume makers, cheese processors, and even coffee roasters turn to this pyrazine to control scent profiles. MIBP delivers its notorious earthy, green, and sometimes earthy-boiled-pea scent at concentrations as low as parts per trillion, making it one of the most potent aroma agents on the planet. Most products contain this chemical as a pure standard or diluted to accommodate industrial blending.
Take a sample of MIBP, and you’ll see a colorless to pale-yellow liquid that releases a strong, sharp aroma even with the bottle closed. Its molecular formula is C9H14N2O, with a boiling point near 118°C at 21 mmHg. The structure places a methoxy group next to an isobutyl chain on the pyrazine ring, producing extreme volatility and high aromatic impact. MIBP dissolves easily in alcohol and organic solvents like diethyl ether or hexane, but not in water. This high volatility and lipophilicity help the flavor bloom in oils and fats but linger only briefly in water-based mixtures. High sensitivity to light and oxygen oxidation means storage always demands tightly sealed, dark, and stable environments.
Manufacturers rarely skimp on purity for industries that depend on reliable standards. Food-grade MIBP typically reaches purity above 98%. Labels break down batch number, CAS registry (123-32-0), and origin. With standards tightening, any residual solvents, heavy metals, or unknown isomers require explicit disclosure in certification documentation. Companies include hazard and risk codes, along with shelf-life guidance if the product needs transport across borders or into strict regulatory regions. Traceable documentation supports recalls or investigations in case anything goes awry further on in the supply chain.
Before modern synthesis, large-scale producers extracted pyrazines by distilling juices or plant material, but that didn’t last. Most today follow a chemical pathway using alpha-amino ketones and suitable catalysts in condensation reactions. Some methods depend on the reaction between isobutyraldehyde and 2-methoxypyrazine followed by refinement through vacuum distillation and column chromatography. Farms and fermentation specialists attempted biosynthesis, using engineered yeast or bacteria, but the cost and efficiency rarely beat chemical methods at scale. For specialty batches, small-batch synthesis still finds a niche among academic labs hunting for rare analogues.
In the lab, MIBP stands up to standard reduction, oxidation, and alkylation reactions. Attempts to tweak its odor profile lead chemists to flank the methoxy or isobutyl with different substituents, hoping to steer the aroma from sharp green bell pepper toward softer earthy notes. Some reactions work well in bench experiments but fall apart under industrial conditions. Oxidizing agents may spoil the subtle structure, while some nucleophilic additions destroy the odor altogether. The parent molecule tends to hang around in natural matrices like grapes or hops, resisting degradation unless exposed to prolonged UV or strong acids.
Labels and scientific references often point to MIBP under several names: 3-Isobutyl-2-methoxypyrazine, IBMP, or Green Bell Pepper Pyrazine. Brands marketing to food processors sometimes call it “Vegetal Essence 123” or “Pea Aroma Compound.” In chromatography catalogs or regulatory lists, it appears by its IUPAC name or CAS number. For research, the pure standard appears under simple abbreviations (MP, IBP) or tied to the supplier’s trade name, adding to the alphabet soup.
Every handler knows MIBP in the lab or plant should stay far from open flames or heat sources. Its intense odor escapes even the smallest crack in a cap, turning the air pungent after a brief exposure. Direct skin or eye contact often leads to irritation, and laboratory staff rely on gloves, goggles, and effective ventilation. Storage regulations demand compliant containers, away from oxidizers and light. Most global workplace standards (such as OSHA in the US or REACH in Europe) call for proper transport labeling, emergency plans, and restrictions on workplace exposure. In the rare event of a spill, staff neutralize with adsorbent and keep the product isolated from waste water or open drains due to high chemical persistence.
MIBP threads its way through every aisle of modern food and beverage development. Winemakers and brewers use it to control the herbaceous character of their products. A slice of fresh bell pepper or the “clean” aroma in some sauvignon blanc owes much to this molecule. In the flavor industry, MIBP helps craft vegetable broths, snack seasonings, and even toothpaste flavorings that suggest cool greens. Perfume and air care sectors lean on pyrazines to build natural, outdoorsy notes. Researchers shifted to study its effect in animal communications, realizing that rodents deploy similar molecules as warning or attraction pheromones. Even in environmental monitoring, labs test for trace levels of this pyrazine in soils and food storage facilities to check for spoilage or contamination.
The last few years saw universities dive into gene editing in crops to change the pyrazine profile—either dialing back the green notes in grapes for certain markets or boosting them for vegetables with a stronger identity. Analytical chemistry labs refine extraction and quantification techniques to measure MIBP down to parts per trillion in wine and food, helping producers avoid “overly green” character. Innovations in biosynthesis chase ways to produce MIBP in yeast or bacteria with greater control and fewer pollutants, although commercial adoption drags behind bench breakthroughs. Tech companies offering synthetic biology tools promise even more precision in targeting MIBP creation or elimination in new plant varieties.
Over the decades, regulatory agencies and toxicologists double-checked what happens when people inhale, eat, or come in contact with MIBP. Low acute toxicity in animal models lets food technologists work with confidence, but the real story sits in chronic exposure and trace accumulation. At high concentration, the compound irritates mucous membranes and may cause headaches or nausea, although few cases reach these levels outside the lab. No conclusive evidence suggests carcinogenicity or organ toxicity in regulated doses, but agencies continue reviewing data as analytical sensitivity improves. Disposal and exposure guidelines remain strict due to odor impact and persistence in wastewater streams, not human health risk.
Looking forward, MIBP’s story spins out into both food and environmental technology. On one front, winemakers and brewers push for ultra-precise aroma control to win over new markets or stamp out vintage flaws. In agriculture, flavor engineers want to design “neutral” or “bold” varietals to please fast-changing palates. Biotechnology walks a thin line, trying to engineer desirable pyrazine levels in staple crops while keeping lobbying groups and regulators satisfied on safety and transparency. Meanwhile, environmental programs explore rapid testing to spot spoilage in bulk storage or trace tainted soil. As digital analysis and synthetic biology both advance, the challenge remains: keep the benefits of MIBP while sidestepping the pitfalls of overexposure or ecological persistence.
Step into any produce aisle, and you’ll catch a scent so sharp it borders on earthy-sweet, even at arm’s length. That crisp green bell pepper flavor comes from a molecule with a name as complicated as its effect: 2-Methoxy-3-Isobutyl Pyrazine. It’s one of those rare chemicals that shapes our experience with food, both in tiny kitchen moments and on big grocery shelves. Despite being nearly impossible to pronounce, it carries punch in tiny doses—think a few parts per trillion, and suddenly a product smells like vegetables straight from the garden.
This chemical pops up naturally in green peppers, asparagus, even Sauvignon Blanc wine. It’s the reason some people detect an unmistakable, almost grassy bite in a glass of white. Over time, food scientists figured out how to reproduce its effect to transform foods, drinks, and even perfumes. You’ll see 2-Methoxy-3-Isobutyl Pyrazine listed as a “flavoring agent” in processed foods, savory snacks, and, on rare occasions, candy that needs a green twist.
People care about taste—sometimes more than they realize. Research suggests smell shapes much of what we call flavor. When a seasoning blend needs to taste “bell pepper fresh,” just sprinkling dried powder doesn’t cut it. 2-Methoxy-3-Isobutyl Pyrazine steps in, bringing the intensity without squishy texture or unpredictable moisture. In the wine world, vintners monitor how much of this molecule sits in grapes. Too much, the aroma comes off like canned peas. In careful balance, it delivers what wine professionals call “typicity,” the fingerprint that shouts, “This is Sauvignon Blanc.”
For winemakers, this molecule’s presence can make or break a vintage. Some people taste it as fresh and exciting, while others find it grassy or even unpleasant. I’ve tasted New Zealand whites that make you pause—the flavor sweeps in with a snap of green vegetable mixed with tropical fruit. Critics argue about whether that’s a good thing. Still, it creates identity. In foods like gourmet popcorn or cheese, careful use brings depth. Too much, and you drift into odd territory, where something as simple as potato chips suddenly hints at garden soil.
Problems start when this molecule overwhelms other notes. In food factories, precision wins the day. Too strong, and instead of a fresh, natural aroma, you get something harsh and artificial. The fix starts with calibration—smaller batch tests, feedback from tasters, and technology that controls how much goes into a recipe. For grapes and vegetables, growers adjust sun exposure and water to manage natural levels. On the flavor-development side, blending with sweeter or citrus notes often smooths the effect.
2-Methoxy-3-Isobutyl Pyrazine reminds us that big changes start small. A few molecules flip a snack from bland to addictive or shift a wine from mild to memorable. Today’s flavor culture seems obsessed with naturalness and experience. Chemists, chefs, and regular folks all chase the right mix of familiarity and surprise. Getting that mix right doesn’t always mean finding new ingredients; sometimes it’s about respecting and carefully wielding the ones we’ve already met, even if only through a bite of something fresh.
Walk through a farmers market and find a table piled high with glossy green bell peppers. Take a deep breath, and there’s a crisp, almost earthy tang to them. That sharp, green punch lines up with 2-Methoxy-3-Isobutyl Pyrazine—often called “IBMP” for short. This little compound carries one of the world’s most divisive scents. Anyone who cooks regularly or bites into a fresh pepper knows this smell. IBMP puts an unmistakable stamp on vegetables, wine, and even some coffees.
IBMP smacks of raw, cut grass and freshly snapped green beans. People pick up on crushed green jalapenos and leafy stalks. There’s a trace of earth after rain and the barest trace of something smoky. In winemaking, IBMP makes itself known, sometimes too much so. I once opened a bottle of Cabernet Franc from the Loire. The bell pepper hit so hard, my dinner guest swore it was pepper soup disguised as wine. Some value this profile, considering it a sign of a wine’s ‘greener’ youth or classic character. Others wish it away, seeing it as harsh or “underripe.”
The concentration of IBMP often stems from cool growing seasons. Grapes and peppers both struggle to shake off this flavor when the sun doesn’t show. Vineyards fighting a cold, wet year will see IBMP levels rocket. Winemakers talk of “pepper bombs” and spend endless hours working out trellising or leaf thinning. I’ve heard vintners trade stories about their green-flavored vintages, some laughing, others swearing off planting those varieties again.
My own kitchen splits down the middle. My mom would fry green peppers, setting off that scent of IBMP through the house and calling it comfort food. My dad would gag, muttering it ruined everything. There’s science for why people are split. Even in tiny amounts—parts per trillion—IBMP jumps out, stubbornly strong. Some folks have a genetic aversion, so what seems “pleasantly grassy” to one seems “raw and vegetal” to another.
Digging deeper, this compound turns up outside the fields. Not just in wine and peppers, but snap peas, asparagus, even coffee beans picked too early. Coffee tasters, or Q-graders, mark a greenish note as a flaw often tied to IBMP. Brewers struggle to scrub it out, sorting beans and tweaking roasts. Letting fruit ripen on the vine slashes IBMP, so growers angle for a perfect picking window.
To tamp down IBMP in crops, careful canopy management in the vineyard helps. Pulling leaves early, letting sunlight through, works wonders. In peppers or peas, letting vegetables fully ripen on the plant makes a difference. Tech can step in—gas chromatography, for example, helps measure and manage these traces at the molecular level. Sometimes, winemakers blend away strong lots or craft their harvests to avoid green edges. At the end of the day, it’s a balance. Some want the crisp edge, others run from it. Learning where to find and fix this flavor gets you a better, more personal food or wine experience.
There’s this molecule, 2-Methoxy-3-Isobutyl Pyrazine (MIBP), that pops up in lots of food science conversations. Bit of a mouthful, but what it brings is a distinct, earthy bell pepper scent. You’ll sniff it in Sauvignon Blanc, green peas, and even in some coffee blends. A dash of a compound like this can really change the feel of a flavor profile, which is why food scientists regularly talk about MIBP.
Whenever anything is added to what we eat, questions come up. We don’t just want our snacks and drinks to taste good; we want to trust what goes into our bodies. MIBP catches attention because its aroma is detectable even in tiny amounts, down to a few parts per trillion. My own first memory of its smell comes from uncorking certain wines in a tasting class—just a drop made all the difference. That strength raises eyebrows about risk, especially since many folks worry about the long names in ingredient lists.
You don’t need lab goggles to notice scientists have had their eyes on food pyrazines for decades. MIBP naturally shows up in vegetables and fruits; we eat and drink it regularly without knowing. Studies keep confirming that—even at levels used to tweak flavors—MIBP poses no health hazard. The European Food Safety Authority (EFSA) and the U.S. Food and Drug Administration (FDA) both listed MIBP as safe. Both base that on toxicity studies, animal testing, and real-life consumption data. Basically, unless someone starts chugging gallons of pure MIBP (not likely), there’s little for the rest of us to sweat about.
Just because something is safe doesn’t mean everyone likes it. I’ve met wine drinkers who love the green-pepper notes and just as many who pour their glass down the drain. High concentrations of this compound can overwhelm. On top of that, some people worry about hidden sources or stacking up flavorings from different foods.
People want their food labeled, and they want to know which flavors come from the garden or a chemist’s bench. Sometimes the only way to mimic a natural note, especially after processing removes originals, means using molecules like MIBP. Regulators keep tabs to ensure flavor chemists play by the rules, sticking to safe levels. As a consumer, I look for transparency from both small-batch producers and giant snack companies.
Trust builds through open information. Listing the presence of added flavors, keeping concentration well within safe ranges, and explaining the role of each compound all help. Food tech isn’t about slipping things past people; it’s about balancing tradition, taste, and safety. Industry can offer sample reports or explain sourcing at the request of retailers or curious buyers. Regular monitoring and research mean that if any genuine risk emerges, changes get made.
There’s plenty of caution built into the system. It’s fine to ask about complicated names in the products we buy, but for MIBP, the evidence stacks up on the side of safety. As long as companies keep things clear and stay within those studied limits, that hint of green pepper is nothing to worry about.
2-Methoxy-3-Isobutyl Pyrazine packs a punch. A tiny pinch changes an entire sensory experience, turning bland into “Whoa, that’s green.” Anyone who has worked with flavors or fragrances knows how something so minuscule can take over a product quick. Most people probably recognize this compound even if they’ve never heard its name; it's the aromatic secret in green bell peppers, Sauvignon Blanc wines, some coffees, and certain chocolate confections. Overdo it, and flavors start to drift into “vegetal” and “earthy” territory – sometimes great, sometimes not the target.
Listen to folks who've struggled to balance that signature note. In beverages, concentrations of 2-Methoxy-3-Isobutyl Pyrazine show up at astonishingly low levels — even fractions of a part per billion (ppb) can be too much. In wine, only about 1–2 ng/L (nanograms per liter) turns a neutral Sauvignon Blanc into something unmistakably grassy. In flavored foods or candies, you're rarely looking for more than half a microgram per kilogram. Scent formulators often stop long before reaching even that level.
I remember a baking trial once. We tried using green bell pepper aroma to give a modern twist to a simple shortbread. The first batch used a solution at 0.05 ppm. Nobody welcomed the cookies. The aroma dominated, dizzying and strange. The lesson stuck: this molecule offers little room for error. Testing began at one-tenth that amount, and only then did complexity peek through rather than shouting over everything.
Why bother with such a delicate tool? The answer hides in the nose. At micro-doses, sensory brightness emerges — the background note in chocolates, the snap in pea purée, the freshness in certain gins. Overshooting turns excitement into confusion. Even in perfumery, concentrations higher than 0.01% rarely serve a purpose. Consumer studies consistently show that even a slight nudge above the recommended range annoys more than it intrigues. That fine line separates a subtle twist from a product nobody buys twice.
For food, work below 1 ppb for most applications. Drinks like wine and spirits thrive around 1-5 ng/L, closer to nature’s baseline. Perfume and personal care formulas usually drop to the 0.001–0.01% range by weight — that’s about 10 to 100 ppm at most, and many creators stay shy of the upper edge. If the note starts to crowd out others, you already shot past the sweet spot.
Books and research can point to numbers but working with a panel changes everything. A tiny increment flips the switch from “that’s fresh” to “that’s all I taste.” Regular sensory checks mean less risk of going overboard. Every producer who’s been burned by an enthusiastic pour learns this truth: more is almost never better.
Start at the lowest detectable level. Blend, taste, wait. Always dose up in tiny steps, using controls and references. If regulations offer guidance, follow them — but always trust the sensory panel over the spreadsheet. For new applications, partner with someone who’s wrangled these compounds before. And keep a record, because it only takes a single drop to remember what too much feels like.
2-Methoxy-3-Isobutyl Pyrazine can surprise you with its power. Even at low concentrations, one whiff reveals why it’s so prized in food science and perfumery. This stuff packs an unmistakable punch, calling to mind green bell peppers, dirt after rain, that earthy note you find in cabernet sauvignon. But strong odors translate to high volatility—and that means treating every bottle with real respect.
I remember the first time I opened a tiny vial in a sensory lab. The aroma escaped instantly and wafted through several rooms. Someone from down the hall wandered in, wondering who had raided the garden. That experience etched the lesson: store these pyrazines carefully or everyone in the building gets an uninvited chemistry lesson.
Pyrazines, including this one, wish for warmth, but they go off quickly with heat and light. Keep them cool and shaded, tucked away in glass containers with a tight seal. I always opt for amber-colored bottles, ideally stored under refrigeration (often between 2°C and 8°C), because sunlight can speed up chemical breakdown and wreck the delicate aroma.
I’ve seen too many people think a food ingredient means ‘safe’ to splash around. Not true for lab-scale work. Simple cotton gloves won’t cut it. It takes nitrile or latex, along with standard lab goggles. One slip and you’ll smell like green peppers for weeks and risk skin irritation. Ventilation matters just as much—an open bench quickly becomes a stink zone. Fume hoods and closed systems save your sanity and your sense of smell.
Every bottle needs a clear label, listing full chemical names and date received. Hazmat symbols help in a shared lab. More than once, I’ve fished an unmarked vial from a fridge only to scramble for an SDS sheet. You don’t want a guessing game on your hands in the middle of a project.
It’s easy to underestimate how a drop spreads across a bench or floor. Paper towels spread the smell around, so use absorbent pads designed for organic solvents. Seal the waste in a plastic bag and get it to chemical disposal—don’t toss it in the regular trash. Wash skin thoroughly if you get splashed, and air the room out for a couple of hours. A little care upfront saves a long headache later.
Many labs thrive by sticking to a simple rule: keep strong-smelling compounds refrigerated, away from light, in tightly sealed bottles, and out of reach unless you’re ready to handle them properly. I’ve found basic habits, like wearing gloves, using fume hoods, and labeling everything, work far better than any clever shortcut. Share safety tips with new colleagues, and always have a cleanup plan before you ever uncork that vial.
If you take a practical approach, pay attention to details, and keep a healthy respect for the compound, even notoriously smelly pyrazines start to seem like just another tool in the kit—one that gives a wine its magic or a product its signature aroma, without turning the workplace into an accidental garden patch.
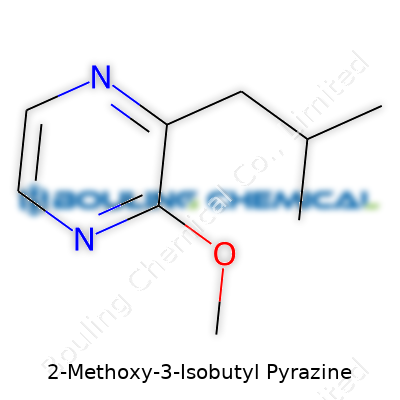

| Names | |
| Preferred IUPAC name | 3-Isobutyl-2-methoxypyrazine |
| Other names |
2-Methoxy-3-isobutylpyrazine Isobutyl methoxypyrazine 3-Isobutyl-2-methoxypyrazine Bell pepper pyrazine IBMP |
| Pronunciation | /tuː ˈmɛθ.ɒk.si θriː aɪ.səˈbjuː.tɪl paɪˈreɪ.zin/ |
| Identifiers | |
| CAS Number | 104-99-4 |
| Beilstein Reference | 1816191 |
| ChEBI | CHEBI:136731 |
| ChEMBL | CHEMBL504901 |
| ChemSpider | 141482 |
| DrugBank | DB14045 |
| ECHA InfoCard | 03d8fa06-18fd-468a-872b-7863f01c9cfc |
| EC Number | The EC Number of 2-Methoxy-3-Isobutyl Pyrazine is 259-900-9 |
| Gmelin Reference | 641066 |
| KEGG | C09662 |
| MeSH | D019281 |
| PubChem CID | 71141 |
| RTECS number | UW8570000 |
| UNII | O60341SE3K |
| UN number | UN3271 |
| CompTox Dashboard (EPA) | DTXSID00879098 |
| Properties | |
| Chemical formula | C9H14N2O |
| Molar mass | 167.23 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | powerful, green, earthy, bell pepper |
| Density | 0.98 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.61 |
| Vapor pressure | 0.01 mmHg (25 °C) |
| Acidity (pKa) | 15.20 |
| Basicity (pKb) | 1.44 |
| Magnetic susceptibility (χ) | -68.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4540 |
| Viscosity | 4.90000009536743 cP (20°C) |
| Dipole moment | 2.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 451.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -62.48 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5920.4 kJ/mol |
| Pharmacology | |
| ATC code | R07AB02 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P501 |
| Flash point | 58°C |
| LD50 (median dose) | LD50 (median dose): >2000 mg/kg (rat, oral) |
| NIOSH | SN8900000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-Methoxy-3-Isobutyl Pyrazine is not established. |
| REL (Recommended) | 0.01 ppb |
| Related compounds | |
| Related compounds |
2-Methoxy-3-sec-butylpyrazine 2-Methoxy-3-isopropylpyrazine 2-Methoxy-3,5-dimethylpyrazine 2-Methoxy-3-n-butylpyrazine 2-Methoxy-3-ethylpyrazine |