The story of 2-Mercaptothiazole started back in the early 20th century, tracing roots to the surge in demand for better rubber products. Chemists searching for new approaches to boost vulcanization found that thiazole derivatives did the job like nothing else. This compound changed the way tires, seals, and gaskets stood up against wear and heat. Old technical books from the 1930s dug into these sulfur-containing heterocycles, picking apart their structure and capabilities, and since then, 2-Mercaptothiazole has lived through decades of industrial change. It may not grab headlines like bigger molecules used in medicine, but its legacy in rubber chemistry is hard to beat.
2-Mercaptothiazole appears as a pale yellow, crystalline solid. You might catch a faint, sulfur-like odor if you bring it up close. It’s known by a slew of trade names and synonyms, like MBT, Mercapto-2-thiazoline, or 2-MBT. Most suppliers stick to white or slightly off-white powders, packaged in drums to fend off moisture. Its lasting value comes from the role it plays, not just in rubber but also as a corrosion inhibitor, and as a building block for plenty of other chemicals. Anyone who’s worked around it knows its gritty, practical impact in large-scale manufacturing.
The physical traits grab your attention: melting point sits around 170-175°C, pretty stable for a thiazole ring. It stays reasonably steady in dry, cool storage. Solubility can be tricky—it doesn’t budge easily in cold water, but solvents like ethanol or acetone can carry it along. It stands up to oxygen and light better than you’d think, offering a longer shelf-life than some related sulfur chemicals. Chemically, it has a reactive thiol group, plus the five-membered thiazole ring, making it a go-to for reactions that need sulfur or nitrogen donors.
Industrial-grade MBT usually comes with clear technical data sheets. Purity will fall north of 98% for most large buyers, but any trace impurities—particularly oxidized forms or heavy metals—have to meet strict cutoffs due to downstream product requirements. TÜV or ISO stickers on the packaging often remind you that suppliers comply with international handling and labeling rules. Labels need to show chemical identifiers, GHS safety pictograms, batch codes, and manufacturer info, following global regulations.
Synthetic chemists have dialed in the process for years. They start with aniline or similar precursors, then cycle through condensation, cyclization, and sulfurization. Industrial recipes balance cost against yield, with hydrogen sulfide or sodium hydrosulfide as key sulfur sources. I’ve seen firsthand how variables like temperature or pH tip the balance between a good run and a messy failure, especially with sulfur compounds floating around. The process rewards experience and precision at each step.
That thiol group doesn’t just sit quietly. 2-Mercaptothiazole jumps into metal binding, making strong complexes with copper, silver, and other metals. This makes it a reliable corrosion inhibitor. It can also act as a key intermediate—alkylation, oxidation, or even ring-opening can modify the parent molecule, creating new agents with special-purpose uses. Some pharmaceutical and agrochemical syntheses rely on such tricks to build larger molecules efficiently, taking advantage of the available reactive sites.
Names change depending on the country or trade group. MBT stands out as the standard shorthand. Other times you’ll spot it as Mercaptobenzothiazole or Benzothiazole-2-thiol, although this edges into a slightly different structural isomer. Safe to say, MBT calls up the same image for most technical buyers and chemists: a vital sulfur compound for tough jobs. In some contexts, manufacturers use brand names or blend numbers, but professionals know to read the fine print for the actual molecular identity.
Working with MBT, you have to watch for dust and skin contact, since it can trigger sensitization or allergic reactions. Personal experience reminds me that a whiff of MBT will stick to your clothes longer than you’d expect, and operators must use gloves, goggles, and proper respiration gear without fail. Storage guidelines say keep it cool and dry, away from acids and oxidizers, with good ventilation. Regulations demand that workplaces install spill kits and emergency wash stations. Companies follow REACH, OSHA, and other standards, performing risk assessments and exposure monitoring to protect workers from its known irritant properties.
Rubber vulcanization takes the lion’s share of MBT use. In factories, you watch it transform ordinary latex into something that lasts millions of miles on the road. Brake pads, conveyor belts, insulation materials—all benefit from MBT as a vulcanization accelerator. Beyond rubber, you’ll find MBT in industrial water systems, forestalling rust and pitting in steel pipes. Lab chemists exploit its metal-binding prowess to make analytical reagents, while pesticide and pharmaceutical makers pursue it for intermediates with unique activities. MBT’s flexibility explains its decades of steady demand.
Research teams dive into MBT’s structure, pushing to reduce its environmental impact and extend its usefulness. Green chemistry efforts focus on making production more sustainable, using recyclable solvents and aiming for closed-loop manufacturing. Some groups aim to replace MBT in rubber with newer, less problematic chemicals, but the search for equivalents that match MBT’s performance without added waste or expense still comes up short. Others look at tweaking its structure for medical imaging or targeting bacteria in food systems. For those in R&D, MBT represents a test case for balancing technical prowess with environmental and health considerations.
Toxicologists have studied MBT for decades, flagging concerns about skin sensitization and aquatic toxicity. It doesn’t break down fast in the environment, so protocols for waste management and accidental release take on real-world importance. Chronic exposure by inhalation or skin contact can cause dermatitis, especially in workers with repeated access. Since MBT can leach into water sources from tire wear and industrial runoff, environmental monitoring picks up any rising levels near plants. Some studies link long-term exposure to possible endocrine disruption in aquatic species, which prompts regulatory agencies to call for tighter controls.
Looking ahead, MBT faces challenges from eco-standards and calls for greener manufacturing. Industry groups push to develop next-gen accelerators with reduced toxicity and easier breakdown. Some markets, especially in Europe, have started phasing out MBT from consumer products where alternatives make sense. Yet, industrial infrastructure around the world still depends on MBT for critical applications, and total replacement looks years away. Advances in waste treatment and capture technology offer one path forward, as does ongoing research into more biodegradable analogues. As economic growth in emerging markets spurs new tire and rubber demand, MBT’s role as a workhorse chemical continues, balanced against the drive for safer, cleaner practices in the decades to come.
Few folks outside of manufacturing know about 2-Mercaptothiazole, but the products it touches end up everywhere. My first taste of its impact came in a tire plant, where the process of making strong, tough rubber felt more like artistry than science. In these settings, the chemical helps “cure” rubber—essentially, it’s like baking bread, except instead of yeast, you’ve got pressure, heat, and a tight timeline. 2-Mercaptothiazole acts as a rubber accelerator, making the sulfur crosslinks that give tires and conveyor belts their grip and bounce snap into place faster. No accelerator, no factory rhythm; jobs slow down, and so does output.
Metals exposed to water and air usually end up rusted or corroded sooner than later. Shipyards and machinery shops throw a lot of effort at this problem. Using 2-Mercaptothiazole as a corrosion inhibitor in metalworking fluids, folks can save engines and high-value parts from wearing out or seizing up. Most mechanics don’t spend time wondering which chemical keeps their coolant from eating its own pipes; they just know that some fluids smell faintly nutty, and those are the ones with 2-Mercaptothiazole doing the quiet work of keeping machines alive.
In factories packed with moving parts, oils and coolants travel through miles of tubes and valves. Breaking down means everything halts: compressors overheat, bearings fuse, equipment fails. Manufacturers mix 2-Mercaptothiazole into lubricants and coolants, not for lubrication, but to shield the inside of pipes from corrosion. This extends the lifespan of gearboxes and keeps the lines running without costly hiccups. Old hands in plant maintenance can often trace longer machine life back to hidden chemical tweaks like this.
Purifying water for factories and home use always demands an ongoing fight against scale and rust. Years ago, I watched a wastewater treatment engineer explain how even small amounts of 2-Mercaptothiazole in closed water circuits make a difference. They use it in boilers and cooling towers to block metallic ions from running wild. That means cleaner water, fewer unexpected shutdowns, and less need for expensive pipe replacements.
With all this utility, balancing benefits and risks matters. Scientific reviews point out that 2-Mercaptothiazole raises concerns for skin allergies and possible toxicity to aquatic life when it enters wastewater. Regulations limit how much can go into each product and how factories dispose of their leftovers. Safer work practices—gloves, ventilation, tailored handling procedures—protect workers. More plants invest in on-site wastewater treatment or switch to formulations with safer profiles where feasible.
As industry pressures mount, options like green chemistry and safer substitutes rise in importance. Some companies now invest in research for alternative accelerants or eco-friendlier corrosion inhibitors, following both regulations and customer demand. Switching over isn’t fast—it means adjusting equipment, training teams, and retesting products for every possible use case.
In factories, workshops, and water treatment plants, 2-Mercaptothiazole keeps showing up as the small ingredient doing a big job. That invisible hand keeps wheels moving, pipes clean, and machines running, so long as risks stay managed and the search for safer options keeps rolling forward.
Handling 2-Mercaptothiazole in a lab or factory throws up more risks than many expect. The compound, used in anything from rubber production to corrosion inhibitors, carries toxicity that wears on both skin and lungs. From direct experience, a single whiff—no proper ventilation—brings about a sharp eye and throat irritation that isn’t easy to shake. Absorbing this chemical through the skin can spark allergic reactions or, over time, harsher symptoms. In some cases, folks complain about headaches and breathing difficulties. Even a minor spill, left for cleanup with bare hands, lands a person in the nurse’s office faster than most realize.
The National Institute for Occupational Safety and Health (NIOSH) places strict time-weighted exposure limits on compounds like this for a reason. Prolonged contact or breathing in the dust raises the risk of kidney or liver damage, according to published toxicology studies. 2-Mercaptothiazole also falls into a class of chemicals under review for carcinogenic effects, stressing extra vigilance.
Cotton gloves or a disposable gown just don’t cut it around this chemical. Chemical-resistant gloves—nitrile or butyl rubber—offer the right barrier, and goggles keep stray dust and splashes out of the eyes. A face shield ramps up safety if there’s a chance of a spill or pressurized process.
In my old chemistry lab, nobody worked with such compounds without long sleeves, a chemical apron, and a pair of boots that didn’t have canvas tops. We learned early that regular lab coats soak up vapors, so we used impermeable materials. Respirators with an organic vapor cartridge became standard any time there was risk of inhaling dust.
Ventilation makes all the difference. Trying to work with 2-Mercaptothiazole on an open bench, windows closed, pushes fumes right into the breathing zone. Hoods and local exhaust fans keep air safe, drawing away vapor and limiting what ends up in the lungs.
Facilities with regular chemical handling use air monitors to track hazardous compounds in real time. On one occasion, our monitor registered an uptick in airborne concentration after a container lid fell under a bench. Shutting down the process and cleaning up quickly kept levels down, underlining the value of continuous monitoring.
Containers get sealed tight after every use. Nobody leaves a workbench with bottles open—vapors travel farther than expected, especially on humid days. 2-Mercaptothiazole stays away from strong oxidizers, acids, or sources of ignition. Storing chemicals on labeled, spill-proof trays cuts down the mess and risk from leaks.
Spills get priority. The right spill kit—a mix of absorbent pads, neutralizers, and thick gloves—makes sure cleanup finishes before the chemical spreads. Any contaminated clothing gets removed and washed separately, never with street clothes.
Facilities track waste closely. Disposal must fit local hazardous waste protocols, with no shortcuts. Dumping it into regular trash or sinks leads to environmental problems and potential fines. Coordinators audit both documentation and disposal regularly, not just during inspections.
Regular training sticks with people longer than a written manual. Watching someone demonstrate proper glove removal and the correct way to label a waste bottle actually builds muscle memory. Regular drills, like what to do if someone gets exposed or how to flush a chemical burn, should stay part of the routine.
Handling 2-Mercaptothiazole with care never stays just theory. Lapses slip through when people forget, so reminders and peer checks help everyone walk out healthy at the end of the day.
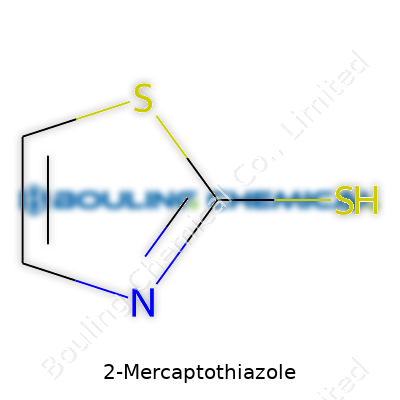
A chemist spends a lot of time staring at molecular diagrams, sniffing around bonding and arrangement. 2-Mercaptothiazole draws interest for more than just its tongue-twister name. Get right into it, the molecular formula is C3H3NS2. That formula breaks down to three carbon atoms, three hydrogens, one nitrogen, and two sulfurs hanging together in a compact structure.
Digging into the structure, you spot a five-membered ring. It holds both sulfur at position one and nitrogen at position three. The “mercapto” group means a sulfhydryl (-SH), stuck on the second carbon. This configuration isn’t just academic. It shapes how the molecule behaves and where it finds its use. For folks who learned organic chemistry by scribbling on paper, it’s a simple enough ring, but packing in both nitrogen and sulfur gives the ring a punch when it lands in chemical reactions.
Chemicals like these don’t exist in a vacuum. 2-Mercaptothiazole pops up in major industries—vulcanization of rubber takes the lead. Rubber needs cross-linking to become tough enough for tires or seals. The sulfur atoms in this compound help glue rubber molecules together. I’ve worked in a lab where the simple mixing of this substance changed the entire character of rubber. Before, soft and sticky. Afterward, durable and reliable.
There’s a broader tale, too. This compound shows up as a corrosion inhibitor. Steel manufacturers have used it to protect pipes and other metal work. Hydrogen sulfide, water, and steel don’t usually get along; without something to calm the mix, pipes corrode fast. Add 2-Mercaptothiazole, and you can see the slower rusting, often by simply monitoring weight loss on sections of test pipes. Its effectiveness wasn’t just theory. Factories running old water systems found the stuff helped add years to infrastructure.
Any strong chemical brings both benefits and challenges. Safety data shows that 2-Mercaptothiazole can irritate skin and eyes. Workers handling the powdered form in manufacturing settings use gloves and face masks for good reason. Over the years, plenty of efforts aimed at reducing inhalation, spills, and runoff came from bad experiences or concern for the environment.
Some push goes toward improving handling: better ventilation, enclosed mixing, personal protection, real-time air monitoring. In research, I’ve seen small spills spark fast, careful cleanup—not just for safety, but to avoid getting strong smells through a whole building. Down the line, companies could explore alternatives—greener corrosion inhibitors, or even new synthetic rubber additives—rooted in recent advances in green chemistry. Already, academic groups run trials, swapping out old chemicals for enzymes or plant-based options that work as well, just with less health risk.
With its unique ring structure and the power of that sulfur-nitrogen combo, 2-Mercaptothiazole keeps making its mark in industry. Demand runs high in the automotive and manufacturing worlds. Newer approaches to safety and greener chemistry matter more with every year. Teaching about it in classrooms prompts real questions about chemical safety, environmental responsibility, and innovation. With the facts and formulas in place, how professionals act—on lab benches, in factories, and inside boardrooms—shapes the legacy of this little thiazole ring for the next generation.
Anyone who’s spent time around chemical syntheses can tell you: some compounds love to surprise. 2-Mercaptothiazole is one of those — a chemical used in rubber manufacturing, corrosion inhibitors, and lab work, known for its sharp odor and toothy bite if handled carelessly. I remember the first time this stuff crossed my desk. Even with gloves and a fume hood, a whiff told me I’d better double-check every seal and label.
Chemicals with sulfur and nitrogen in their structure can act up if you don’t store them right. 2-Mercaptothiazole, in particular, breaks down under heat, light, and damp conditions. Letting it clump up next to a heat source or under sunlight isn’t just lazy — it’s a gamble that can end with hazardous gases in the air or a full-blown cleanup headache.
More than a bad smell, this stuff can irritate the skin, eyes, and lungs. Spilled powder won’t just stay where it lands. Static, movement, or even a heavy sigh can make it airborne, putting people at risk without warning. That’s a reminder nobody forgets twice.
Safe storage starts with a cool, dark, and dry location. Don’t just shove it on any shelf — keep it in tightly sealed glass or high-density polyethylene containers. Once, someone on my team gave in to convenience, leaving a bottle on a window-ledge. The label faded, the cap weakened, and soon, we traced a foul smell back to that one lazy choice.
Label every container boldly with the full chemical name and date received. Some outfits use color code systems to make dangerous materials impossible to mistake. I’ve seen labs using nothing fancier than colored tape and bold markers, but that system saved time and nerves more than once.
Never store this chemical near oxidizing agents or acids. Reactions can spark off much too easily if containers fail or if powders mix. Anyone who’s cleaned up after a tiny lab fire knows just how quickly things snowball from “minor leak” to “full evacuation.”
Even sealed, volatile compounds like this benefit from storage in a well-ventilated chemical cabinet — ideally one built to handle corrosion. Frequent checks for leaks or residue around lids make a big difference. I’d recommend an inventory log for dates and inspections, both for safety and to avoid nasty surprises from aging stockpiles.
Personal protective gear isn’t only for those working directly with the chemical. Anyone checking or moving stock should wear gloves, goggles, and masks. I learned early that casual encounters with old chemical dust can mean a rushed trip to the eyewash station, and that’s better avoided entirely.
Disposing of 2-mercaptothiazole needs proper hazardous waste procedures. Pouring leftovers down the drain or into the regular trash doesn’t just break rules—it risks public safety and the environment. From firsthand experience, the cleaner the disposal record, the fewer headaches from regulators and the community.
Local authorities and trained waste professionals can guide the right way to get rid of old or spilled material. If in doubt, ask. Playing guessing games with hazardous waste has bitten plenty of good chemists.
Care for people and the environment drives the right storage of 2-mercaptothiazole. Every measure, from good lighting in the chemical storeroom to regular reminders about PPE use, adds up to fewer accidents and cleaner workspaces. The stories I’ve collected over the years share a theme: it only takes one shortcut to make a routine job into a crisis. Vigilance pays for itself every time.
2-Mercaptothiazole turns up in a lot more places than most people realize. Often used in rubber processing, it keeps industrial products flexible and helps prevent early breakdown. Anyone near manufacturing lines or working with processing chemicals has probably encountered this compound, maybe without even knowing it.
Workers who breathe in 2-Mercaptothiazole sometimes feel it almost right away. The smell alone—a biting aroma that stings the nose—can tip you off. But the real problem starts beneath the surface. Eyes begin to water, throats itch, skin turns red and patchy. These aren’t just minor annoyances. Occupational safety agencies have traced rashes and allergic reactions back to this chemical. I’ve talked to folks who had to leave the floor after just a few hours because their hands broke out in blisters. This points to serious skin sensitivity, which can persist and even worsen with repeated exposure.
Exposure to 2-Mercaptothiazole doesn’t stop at surface irritation. Inhalation has been linked to headaches, dizziness, and nausea. For folks spending months or years in poorly ventilated areas, these symptoms often turn chronic. The U.S. National Library of Medicine and CDC have raised red flags about respiratory problems that crop up with regular contact. Respiratory irritation can lead to asthma-like symptoms, especially in people who already struggle with allergies or lung issues.
Skin absorption raises another alarm. I’ve read about workers developing allergic contact dermatitis—after direct contact with machine lubricants or gloves treated with the compound. This reaction often lasts long after the first incident. In fact, a few manufacturing plants have had to add extra medical checks just to catch early warning signs before employees’ reactions got out of hand. Nobody wants to be out of work for weeks just because of a preventable chemical reaction.
Reliable safety research keeps circles cautious about 2-Mercaptothiazole’s links to cancer. The European Chemicals Agency considers it a substance of very high concern. Some animal studies hint at possible carcinogenic properties, so factories can’t put this risk aside. While a definite link to human cancer isn’t proven, ignoring the warning signs could end up costing lives or at least quality of life down the road. Safety data sheets highlight this potential, making it clear there’s no call for complacency.
From my own time working factory safety audits, the difference comes down to management fixing the basics. Proper ventilation in high-exposure spaces clips down the risk. Personal protective equipment—like gloves and face shields—goes a long way. Not all gloves block these chemicals, so plants need to pick the right kind. Washing stations near production lines help workers rinse off right after handling. Training also helps people recognize symptoms early before it’s too late.
2-Mercaptothiazole brings measurable risk to those who work near it. Companies can invest up front in modern ventilation, regular hazard reviews, and training programs not just for compliance but to protect real people. Industry leaders who put health at the forefront win worker loyalty and avoid expensive shutdowns. Addressing chemical hazards isn’t just bureaucracy or paperwork—it's the difference between thriving teams and chronic absenteeism. Every improvement pays back in safety, productivity, and peace of mind.
| Names | |
| Preferred IUPAC name | 1,3-Thiazole-2-thiol |
| Other names |
2-Mercaptobenzothiazole MBT Benzothiazole-2-thiol 2-Benzothiazolethiol Mercaptobenzothiazole |
| Pronunciation | /tuː mɜːˌkæp.toʊ θaɪˈæzoʊl/ |
| Identifiers | |
| CAS Number | 504-16-5 |
| Beilstein Reference | 1207931 |
| ChEBI | CHEBI:17948 |
| ChEMBL | CHEMBL37975 |
| ChemSpider | 5744 |
| DrugBank | DB11325 |
| ECHA InfoCard | EC#: 202-405-7 |
| EC Number | EC 205-736-8 |
| Gmelin Reference | 8786 |
| KEGG | C01431 |
| MeSH | D004516 |
| PubChem CID | 7000 |
| RTECS number | XN6476000 |
| UNII | 5X59MZ7QOF |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C3H3NS2 |
| Molar mass | 167.24 g/mol |
| Appearance | Yellow crystals |
| Odor | disagreeable |
| Density | 1.42 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.39 |
| Vapor pressure | 0.000168 hPa (25 °C) |
| Acidity (pKa) | 5.4 |
| Basicity (pKb) | 8.53 |
| Magnetic susceptibility (χ) | -59.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.670 |
| Viscosity | Viscosity: 48 mPa·s (20 °C) |
| Dipole moment | 3.75 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 146.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -42.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -364 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | D08AE07 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes serious eye irritation. May cause an allergic skin reaction. Toxic to aquatic life with long lasting effects. |
| Precautionary statements | Precautionary statements: P261, P273, P280, P305+P351+P338, P337+P313 |
| Flash point | 157°C |
| Autoignition temperature | 230 °C |
| Lethal dose or concentration | LD50 oral rat 398 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 398 mg/kg |
| NIOSH | K527 |
| PEL (Permissible) | PEL = "5 mg/m3 |
| REL (Recommended) | REL: 5 mg/m³ |
| IDLH (Immediate danger) | 500 mg/m³ |
| Related compounds | |
| Related compounds |
2-Aminothiazole 2-Mercaptobenzothiazole 1,3-Thiazole Benzothiazole |