Many folks outside the chemical industry probably haven’t given much thought to 2-isopropylimidazole. Its story starts in the middle of the 20th century, a time when imidazole derivatives found new energy in organic synthesis labs worldwide. Chemists were hungry for compounds that could serve as building blocks for new drugs and performance materials. Through trial, error, and a lot of glassware, researchers stumbled upon the practical benefits of sticking an isopropyl group onto the imidazole ring. The result? A molecule with enhanced solubility and a reactivity profile that didn’t make lab techs dread their next purification run. Over the years, interest waxed and waned, but 2-isopropylimidazole quietly earned its place on many synthetic chemists’ shelves.
Big multinational suppliers and boutique chemical outlets both keep 2-isopropylimidazole in their catalogs, offering it in bottles ranging from a few grams to industrial drums. The pure stuff usually shows up as a white to lightly beige crystalline powder. Market demand often comes from specialized polymer production, curing agents, and niche pharmaceuticals. Because buyers range from researchers tinkering with new reaction routes to folks running mid-scale industrial reactions, supply chains keep nimble stock levels and batch consistency high.
This compound comes with a melting point nudging 80-83°C and boils at nearly 300°C, making it a solid choice for higher temperature applications. Its solubility in water doesn’t compare to simpler imidazoles, but it still mixes decently with organic solvents such as ethanol, acetone, and chloroform. The isopropyl group sticks out, lending it a faint musty odour. Imidazole’s alkaline character survives the substitution, so in reactions, it pulls its weight as a mild base or nucleophile. Chemists appreciate its stability under normal handling conditions. Light doesn’t bother it much, and it rarely plays havoc with glassware or storage plastics.
Suppliers label this product with CAS number 2238-12-4. Purity hovers around 98%-99% for research-grade stock, and industrial supplies sometimes relax specs to 97% if the price needs trimming. Moisture and heavy metals stay tightly controlled; most spec sheets highlight ppm levels below industry norms to make customs and quality assurance go smoother. Labeling also spells out storage advice, common hazards, and batch ID numbers for easy trace-back if something goes sideways during use.
The most common lab synthesis relies on a Debus–Radziszewski reaction, mixing isopropylamine with glyoxal and ammonia under mild heating. In production plants, tweaks cut waste and allow continuous processing; for example, using pressure reactors and improved separation technology. The path from basic feedstocks to the final crystal takes patience, but the overall process doesn’t demand exotic equipment. Some newer routes explore catalytic hydrogenation and greener oxidants to shave both time and cost, but the base chemistry hasn’t changed much in decades because it’s stubbornly reliable.
2-Isopropylimidazole steps up in acylations, alkylations, and more exotic cross-coupling reactions. It acts as a backbone for hybrid ligands in transition metal catalysts, and its nitrogen atoms remain open to further functionalization. It handles mild electrophiles and can absorb an additional alkyl or aryl group without losing the core imidazole functionality. Using it as a starting point, chemists have unlocked paths to pharmaceutical intermediates, ionic liquids, and corrosion inhibitors. The isopropyl group doesn’t block all reactive sites, so it allows enough versatility for continued chemistry, but it adds steric heft where that’s helpful.
You might see this compound sold under names like 2-(1-methylethyl)imidazole, or shorthand as IPI or 2-PI. International trade databases stick to the systematic name so paperwork keeps clear, avoiding confusion with close relatives like 2-methylimidazole or isopropylimidazoline.
No matter how safe a powder may look, rules require gloves, goggles, and careful ventilation. 2-Isopropylimidazole isn’t as nasty as some organics, but it can still irritate eyes, mucous membranes, and skin, especially during extended exposure. Dust in the air raises both inhalation risks and flammability hazards, so chemical storage rooms keep it away from open flames and oxidizers. Waste policies classify it with “other nitrogenous organics”, which means extra care in disposal to keep local regulators happy and waterways clean.
Most uses cluster around epoxy resin curing and specialty adhesives. 2-Isopropylimidazole speeds up cure times, creates harder surfaces, and extends the working life of engineered polymers in automotive, aerospace, and electronics. Resins set better, last longer, and take abuse from physical or thermal shock. Beyond materials, some researchers try it out as a starting material for heterocyclic drugs or for fine-tuning agrochemical formulations. A few labs look at its behavior in semi-conductor and optoelectronic materials, too, though that remains at the curiosity stage for now.
Academic and corporate labs keep finding odd new uses for 2-isopropylimidazole. Scientists tweak the molecule to explore new corrosion resistance coatings, or test improved base catalyzed reactions that legacy imidazoles can’t quite manage. Teams looking at “green chemistry” push for alternate manufacturing that avoids noxious solvents and cuts down on off-gassing. Some universities encourage interdisciplinary research, using this chemical as a model system to study basic nitrogen heterocycle chemistry or protein mimicry because its structure offers a middle ground between simplicity and challenge.
Compared to older aromatic amines, 2-isopropylimidazole ranks low for acute toxicity in animal tests. Doses that would trouble a mouse far exceed what workers see in industrial or lab settings. Long-term studies, though, are scarce; chronic effects or subtle environmental interactions still could surprise eventually. The compound’s breakdown products usually degrade under normal wastewater treatment, but some environmental groups advocate more scrutiny since most imidazoles persist just long enough to demand attention. Most countries treat it as “nuisance” level hazardous, not high-risk, which keeps oversight practical yet vigilant.
Where industrial innovation hits a wall with old chemistries, 2-isopropylimidazole steps in as an alternative or improvement. Researchers keep an eye on its ability to anchor tailored properties to larger molecules, whether in flexible electronics, biopharmaceutical scaffolds, or next-generation polymers. Demand from research reactors in Asia and new materials plants in Europe suggests that, if supply lines hold steady, the molecule will keep showing up in patents and production lines. The push for greener processes and better life-cycle profiles could push for renewed interest in its derivatives or methods of synthesis, as chemical manufacturers grind toward lower emissions and less hazardous waste streams, which will likely pull 2-isopropylimidazole and its cousins along for the ride.
Open up a box of medicine, look at the blister packs, and you rarely see unfamiliar chemical names on the label. Still, many people don’t realize how many uncelebrated ingredients work quietly behind the scenes to make those products possible. 2-Isopropylimidazole is one of these. Its name hardly rolls off the tongue, and unless you work in a lab, you probably haven’t thought much about it. Still, this compound plays a pretty interesting role across several businesses, especially where polymers and plastics shape everyday life.
In the world of manufacturing, workers use 2-isopropylimidazole as a curing agent. Epoxy resins need something to “set” them, much like how concrete needs water. Instead of just hardening for its own sake, resins depend on curing agents to create those super-strong chemical bonds that stand up to heat, water, and time. 2-Isopropylimidazole steps in to do just that. Its molecular structure reacts neatly with common resin blends, turning gooey coatings into a rock-solid finish.
Beyond factory floors, you’ll find the results in construction, electronics, cars, and more. Adhesives that hold things together often rely on the tough results made possible thanks to this compound. In circuit boards, that glassy green finish called “solder mask” shields delicate wiring. Makers coat solar panels, wind turbines, and countless other products with epoxy made tougher using chemistry like this.
Think back to doing home repairs with a two-part glue, or smelling paint touch-ups at the office. Epoxies make all that possible, but to really work, they need the strength from curing agents such as 2-isopropylimidazole. Strong chemical bonds—built through reactions triggered by agents like this—mean floors can take a beating and circuit boards stay resistant to water and heat.
The electronics industry especially relies on this compound because reliability cannot be left to chance. Laptops, mobile phones, and even the latest electric cars need connections that keep working after years of vibration and temperature swings. Using reliable curing agents helps reduce failures. Better materials mean longer device lives and less toxic waste from discarded gadgets.
Take it from someone who once helped out in a small-scale electronics workshop—there was always a hunt for the magic formula that would give consistent, reliable bonds. One batch of glue would harden too brittle, another too tacky. Getting the mix right with proper curing agents could save hundreds in wasted time. Companies work with ingredients like 2-isopropylimidazole to fine-tune their products, so end users don’t run into sticky (or not sticky enough) problems down the road.
On top of that, better-curing materials help manufacturers sell products that work in tough conditions, like airplanes and wind farms. Nobody wants parts failing mid-flight or in a storm. Focused research into chemical agents, backed by real-world testing, helps keep these products safe and functional.
No chemical comes without questions. There’s focus these days on worker safety, responsible sourcing, and reducing environmental impact. Plant managers, chemists, and regulators keep a close eye on handling and disposal, since, like many industrial chemicals, improper use can cause problems. Many companies look for greener alternatives, but so far, nothing quite replaces every feature that compounds like 2-isopropylimidazole provide.
For now, as new industries grow and demand stronger, longer-lasting materials, interest in this compound won’t fade fast. Technical workers continue tweaking their recipes, while others work in labs finding safer substitutes. Until then, much of our modern world quietly depends on the chemistry that happens out of the limelight.
A bottle of 2-Isopropylimidazole never calls attention to itself. It sits, labeled and maybe a bit dusty, on the back shelf of a lab. Easy to forget but quick to bite if kept without thought. Long ago, I learned that short-cutting chemical storage turns manageable risks into real danger. Nobody enjoys the panic of hunting for a spill kit. Even a relatively common compound like this deserves the right space and attention.
Humidity in a storage area turns benign chemicals into a headache. Once moisture creeps into 2-Isopropylimidazole, clumping isn’t the real issue — it’s outright degradation or unwanted side reactions. I’ve watched beads of sweat form on container walls during monsoon months. To avoid this, I always tucked my stock in a dry cupboard, with desiccant packs working overtime. No one wins trying to use reagent that’s half-gone to hydrolysis.
I hear “store at room temperature” and picture all sorts of places — a sunlit bench, a box near a space heater, a shelf by a window. Most labs get hotter than you think, with air conditioners battling equipment that throws off warmth. Direct sunlight cooks bottles, sunlight degrades chemicals, and radiators bake whatever’s unlucky enough to be nearby. Storage for 2-Isopropylimidazole means a spot out of sun, away from heat, in a place you know actually keeps its cool. A digital thermometer nailed to the shelf saved my old lab from tossing a whole delivery of overheated stock.
Fewer things irk me than finding a cap left loose. Even trace vapors from chemicals like 2-Isopropylimidazole smell sharp and off-putting. We once traced a lab-wide stink to a cracked plastic bottle, fumes drifting out, irritating almost everyone. Airtight storage stops escape for both powder and liquid forms. Replacing cracked lids and correctly screwing on closures takes seconds and saves hours cleaning up accidental contamination.
I’ve seen storage mistakes where basic compounds and acids shared a shelf. Unlabeled containers beside open flasks. Mixing up bottles isn’t just forgetfulness; it’s an accident waiting for the right nudge. Separation lowers the odds of dangerous reactions, especially during frantic searches mid-experiment.
Permanent marker labels fade and fall off. Poor labeling leads to “mystery bottles” — a phrase that makes anyone with sense nervous. Nobody wants to gamble on whether the white powder is what the catalog claims. That means clear labeling with date received, opened, and maybe even batch codes. Good records and clear handwriting save time, money, and nerves.
Old chemicals gather dust — and sometimes throw off byproducts you don’t want. Every few months, I dug out old stock and reviewed expiration dates. Tossing out the expired stuff brought relief and fewer headaches when audits rolled through. Order and tidiness protect the lab, your research, and your coworkers. It’s the simple steps — dry, cool, sealed, separated, and labeled — that keep headaches small and accidents rare. Proper care for something like 2-Isopropylimidazole isn’t overcautious. It’s the grown-up way to do chemistry.
Anyone who’s worked around chemicals knows that names like 2-Isopropylimidazole can mean several things. In the lab, it pops up as a white powder, not much different from a thousand others stacked on the shelves, but there’s more hiding behind its bland looks. Most folks probably haven’t heard of it unless their job involves chemistry or making things that require specialty compounds, but it’s still out there, used in catalysts, coatings, and as part of certain pharmaceutical processes.
Most chemicals come with some sort of warning, and 2-Isopropylimidazole is no different. According to data sheets from several suppliers, this one can irritate skin, eyes, and the respiratory system. Some people shrug off these warnings, thinking gloves and goggles will handle everything. After years of seeing coworkers get careless—and then pay for it with rashes and watery eyes—my respect for those hazard icons grew. Something that burns your skin or messes with your breathing isn’t something to treat lightly.
There’s always someone who wants the hard numbers. Studies suggest that this imidazole derivative has a low acute toxicity, based on animal testing, but “low” doesn’t mean safe for care-free use. Sometimes, toxic effects only show up after years of exposure. Over time, I’ve seen workplaces skip good ventilation, ignore the fine dust left after pouring powders, or treat chemical waste disposal as an afterthought. Those shortcuts catch up eventually.
Run-off and improper disposal usually fly under the radar until fish start turning belly up or regulators step in. Companies that use 2-Isopropylimidazole can’t just pour leftover chemicals down the drain and call it a day. Even compounds that break down easily might still react with other stuff in the environment, creating trouble that’s harder to track.
People far removed from the lab or factory floor might think chemical safety isn’t their problem, but with modern supply chains, a lot of hands touch a product before it lands in stores. Mishandling chemicals during manufacturing can affect water supplies, air quality, or even the finished products we use daily. I remember hearing stories from old-timers in town who worked at plants without proper safeguards—wildlife vanished, and neighbors got sick. That kind of impact doesn’t stay buried for long.
Safe handling beats cure every time. Gloves, goggles, and lab coats matter, but so does simple training. People shouldn’t just memorize the chemical’s name—they should practice spill drills and proper cleanup. Fume hoods and good exhaust fans turn a risky workspace into a safe one. After seeing how fast an accidental dust cloud can fill a cramped room, I won’t work with powders in an unventilated space again.
Proper labeling and storage cut down on accidents, too. I’ve watched trouble start with a poorly capped jar or a faded label no one could read. Getting rid of leftover chemicals should follow local rules, not the path of least resistance. Some places offer pickup programs or designated drop-offs, and it costs far less than dealing with a spill or sudden health crisis.
At the end of the day, 2-Isopropylimidazole doesn’t need to be a villain. Treated with respect, it’s just another tool. Ignored, it turns into a problem nobody wants to face.
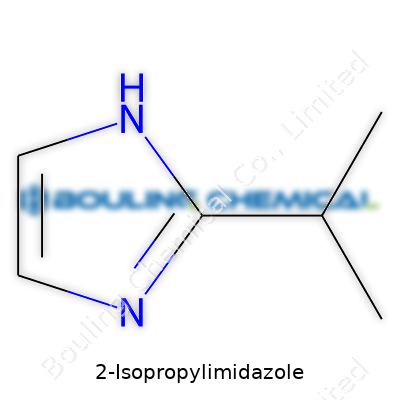
So you’re looking for the basics on 2-Isopropylimidazole. The chemical formula is C6H10N2 and the CAS number is 3220-76-2. That’s the sort of thing researchers and folks in the industry need to jot down before even thinking about firing up their equipment. As simple as these two pieces of information look, they unlock access to a whole world of research, safety data, and commercial supply channels. Miss that part, end up in paperwork limbo, or worse, ordering the wrong thing entirely.
The thing about this kind of imidazole is its role behind the scenes—catalysts, pharmaceutical intermediates, and even some epoxy curing processes rely on it. I spent some time in a lab that dabbled in making specialty polymers, and it amazed me how compounds like this would quietly shape the properties of the end product. Try swapping it out for something else and the recipe just falls flat or unexpectedly gels up. Sure, the chemical structure is simple—an imidazole ring with an isopropyl group—but that’s often the sort of tweak that makes a game-changing difference in industrial chemistry.
No one forgets their first mix-up—once, early in my career, I grabbed an isomer with a nearly identical name and formula. Turns out, a tiny shift in structure changes the whole reaction. Regulatory bodies and suppliers will only trust documentation that spells out both the chemical formula and CAS number. Forget to specify one or the other, you end up searching endless lists, rerunning experiments, or explaining a costly mistake to your supervisor. Accuracy saves time and trouble, especially as chemicals start moving around the globe; traceability starts with these fundamentals.
2-Isopropylimidazole isn’t exactly a household term, but its applications touch everything from drug synthesis to composite manufacturing. Companies want catalysts that speed things up, deliver yield, and keep toxicity in check. This is where C6H10N2 delivers—compared to some legacy options, it can enable reactions at milder temperatures and lower doses. On the other hand, handling and disposal rules keep getting stricter. If industry ignores proper storage or safe work limits, accidents happen, and the chain of trust in supply starts to crack.
Lab supply managers and process engineers who handle 2-Isopropylimidazole face another daily grind—balancing cost, purity, and supply reliability. In one job, I watched a project stall for weeks just waiting for a fresh shipment to clear customs, thanks to a paperwork snag on the CAS number. At that point, chemistry experience takes a back seat; your “bottle-neck” isn’t technical, it’s compliance. Knowing these details up front gives everyone a stronger hand, whether you’re negotiating with a chemical supplier or writing up a hazards assessment for the boss.
What eases the headaches? Reliable supply partners, digital inventory systems keyed in on formula and CAS, and crew training that emphasizes details, not shortcuts. In practice, it pays to keep clear records, update safety data sheets, and work with partners who treat these details seriously. Chemistry moves fastest and safest when everyone on the team can specify exactly what they’re using—down to the molecule. 2-Isopropylimidazole, C6H10N2, 3220-76-2: that’s more than trivia, it’s good practice.
Opening a bottle of 2-Isopropylimidazole isn’t like uncapping vinegar or ordinary paint thinner. This chemical doesn’t draw a crowd, but it slides under the radar and finds a way to cause trouble if you give it a chance. In my own line of work around labs, I’ve seen folks toss empty containers into a regular trash can just to save time, not thinking about where those dregs end up. That kind of shortcut passes risk along to the next person—janitors, landfill workers, sometimes folks you’ll never meet.
The material safety facts point to one thing: direct skin contact or inhaling the dust should be avoided. I once watched a colleague ignore the gloves, ended up with burning hands for several hours, and he didn’t make the same mistake twice. Whatever the use case—synthesizing pharmaceuticals, formulating corrosion inhibitors, working up catalysts—this chemical won’t hand out warnings before it bites.
Use nitrile gloves, not the thin plastic kitchen ones. Forgetting safety glasses just isn’t worth the risk—splashes may be rare, but once is all it takes. Reliable lab coats keep surprises off your clothes and skin. If you expect powder or fumes, bring out the respirator.
The work area should have ventilation. A fume hood isn’t a suggestion—it stands as the real line between a productive day and a trip to the medic. I’ve seen shops try to save money by setting up “temporary” ventilation. In real life, duct tape doesn’t stop vapor.
Spills always happen. Once, someone bumped a flask—fumes filled the air, the next room caught a whiff, and confusion spread. Absorb liquids with spill pads designed for chemicals; scoop up powders using a dustpan and then wipe surfaces with a damp towel. Never dry sweep—raising dust just spreads the danger further.
People underestimate what “trace amounts” can do. Mop up leftovers, and always label the cleanup bag—hazardous waste, not everyday trash. When possible, separate contaminated gear right away, so custodians don’t get a surprise. Schools and research teams should invest in clear instructions on cleanup protocols and drills.
Flushing 2-Isopropylimidazole down a sink isn’t just lazy, it’s actually illegal in most places. Tap water doesn’t magically neutralize what you pour down the drain. Waste treatment systems weren’t built for chemical residues like this. The law steps in hard for a reason: community water sources are at risk.
In practice, every lab or facility should store chemical waste in containers made for hazardous substances, then tie up disposal with a certified waste handler. Tracking manifests and using proper labels keep costs down in the long run and build trust with neighbors and regulators.
Moving forward, plan chemical use tightly. Only open as much as you really need, which means fewer leftovers at the end of a day. Make sure staff all get a run-through with the safety data sheet. If a bottle’s past expiration, don’t try to “use it up”—it likely won’t behave the way you expect.
Every real-world solution starts with respect for the chemical’s risk—and for the people who may eventually deal with leftovers. Treat every step of handling and disposal as if someone you know might pay the price for a shortcut. A safe, methodical approach saves pain, money, and time for everyone down the line.
| Names | |
| Preferred IUPAC name | 2-(Propan-2-yl)-1H-imidazole |
| Other names |
2-Propan-2-yl-1H-imidazole 2-(Propan-2-yl)imidazole Isopropylimidazole |
| Pronunciation | /tuː aɪˈsɒprəˌpɪl ɪˈmɪdəˌzoʊl/ |
| Identifiers | |
| CAS Number | [3228-91-5] |
| Beilstein Reference | Beilstein Reference 120351 |
| ChEBI | CHEBI:84135 |
| ChEMBL | CHEMBL140970 |
| ChemSpider | 65248 |
| DrugBank | DB11298 |
| ECHA InfoCard | 100.069.901 |
| EC Number | 208-164-2 |
| Gmelin Reference | 8776 |
| KEGG | C16512 |
| MeSH | D000382 |
| PubChem CID | 4808 |
| RTECS number | UX7350000 |
| UNII | 08H42F9V41 |
| UN number | UN3276 |
| Properties | |
| Chemical formula | C6H10N2 |
| Molar mass | 110.16 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | characteristic |
| Density | 0.984 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.36 |
| Vapor pressure | 0.0047 mmHg (25°C) |
| Acidity (pKa) | 14.5 |
| Basicity (pKb) | 6.95 |
| Magnetic susceptibility (χ) | -64.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.484 |
| Viscosity | 2.55 mPa·s (25 °C) |
| Dipole moment | 1.89 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 249.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -92.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3895 kJ·mol⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: "P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | Flash point: 113°C |
| Autoignition temperature | 460°C |
| Lethal dose or concentration | LD50 (oral, rat) 970 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 970 mg/kg |
| NIOSH | JN8575000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-Isopropylimidazole is not specifically established by OSHA. |
| REL (Recommended) | 2500 mg/m³ |
| Related compounds | |
| Related compounds |
Imidazole 1-Methylimidazole 2-Ethylimidazole 2-Propylimidazole 2-Phenylimidazole |