Chemists in the mid-20th century saw the promise of isocyanate chemistry, as new polymer branches started to take hold. With the rise of acrylic polymers, companies and researchers hunted for linkers able to bridge organic flexibility with sturdy functionalities. It wasn’t long before 2-isocyanatoethyl methacrylate, also known as IEM, came into play. People familiar with industrial R&D during those years recall spirited debates about side chain modifications. Eventually, IEM carved out its niche—serving in applications where crosslinking, adhesion, and weatherability each played central roles. The development happened alongside improvements across the coating and plastics industries, cementing IEM’s reputation as a specialty co-monomer.
2-Isocyanatoethyl methacrylate combines two highly reactive groups: an isocyanate moiety and a methacrylate double bond. The molecular formula is C7H7NO3, with a molar mass close to 153 g/mol. Most users encounter the compound as a clear, pale yellow liquid. As a raw material, it allows manufacturers to introduce reactive isocyanate groups into acrylic or methacrylic polymer platforms. It’s made available by several chemical suppliers, sometimes under alternative product names, often with purity levels north of 98% when intended for synthesis or specialty coatings.
Dense, acrid, with a sharp odor—that’s how most lab workers describe IEM. It’s heavier than water, tipping the scale with a density around 1.1 g/cm³ at 20°C. It takes heat to make it boil, with a boiling point roughly at 94°C under reduced pressure. The substance remains soluble in most common organic solvents, such as acetone, ethyl acetate, and dichloromethane, while it stays stubbornly immiscible with water—though presence of moisture quickly leads to unwanted side reactions. It exhibits a refractive index close to 1.47 and a viscosity that allows easy blending into reactive mixes. IEM’s dual functional groups create reactivity both toward nucleophiles (via the isocyanate group) and through radical polymerization (via the methacrylate group).
Proper labeling needs to mark 2-isocyanatoethyl methacrylate as hazardous. Material safety data sheets (MSDS) always call attention to its toxicity and sensitization hazards. Most manufacturers market it with clear batch and purity statements, kept tightly capped in brown glass or coated metal containers. Labels include hazard pictograms indicating acute toxicity, skin and respiratory sensitization, and reactivity concerns. Typical specifications demand a refractive index test, acid value check, and GC purity analysis. Those working in regulated environments must follow both local and European REACH or US TSCA labeling standards.
Synthesizing IEM takes finesse. The most widely-used route involves reacting methacryloyl chloride with 2-hydroxyethyl isocyanate in the presence of acid scavengers like triethylamine. The process also gives off heat, so cooling and careful control matter. Some routes flip the sequence, starting from the isocyanate side—using phosgene to convert ethanolamine into isocyanate, then introducing methacryloyl chloride. Both methods require tight exclusion of moisture, or else hazardous by-products like CO₂ and urea derivatives crop up and scuttle the yield. Industrial plants invest real money into handling these raw materials with scrubbers and inert atmospheres, both to keep product quality high and to avoid exposing workers to escaping fumes.
IEM’s chemistry opens doors for creative polymer design. The methacrylate group allows for free-radical polymerization, slotting IEM into acrylic chains, while its isocyanate group reacts with alcohols, amines, or even water to form urethanes, ureas, or polyols. Many advanced coatings take advantage of this by using IEM to add crosslinking to acrylic or hybrid resin systems, improving scratch resistance and adhesion on glass, metal, or plastic. Chemists often fine-tune IEM’s properties through copolymerization, blending with other methacrylate or acrylate monomers to balance flexibility and stiffness. In reactive hotmelt, IEM can bridge between phases, boosting performance compared to standard acrylates or polyurethanes.
2-Isocyanatoethyl methacrylate travels under a few aliases. The common acronym “IEM” pops up in technical documentation and purchasing lists alike. Synonyms include 2-methacryloyloxyethyl isocyanate and β-isocyanatoethyl methacrylate. Large industrial catalogs sometimes list it with designations like CAS 30674-80-7. Other specialty chemical companies brand IEM under specific trade names, but always flag the chemical backbone for regulatory transparency.
Working with IEM means working with a potent irritant and sensitizer. It attacks both skin and mucous membranes, and repeated exposure can lead to asthma-like symptoms. Proper training in chemical hygiene makes a difference. IEM never gets handled outside fume hoods in serious labs, and companies insist on nitrile gloves, chemical splash goggles, and in some cases, full-face shields. Spills get cleaned with inert absorbents—never paper towels that could smolder from reaction heat. Air monitors in plant settings help flag vapor leaks, and spill response teams carry special cartridges designed for isocyanate vapors. Safe storage keeps the compound cold, dry, and away from direct sunlight.
IEM holds real value in the specialty coatings world. Drawing on experience with polymeric materials, it becomes clear that introducing isocyanate functionality directly into methacrylate chains delivers advances in toughness, chemical resistance, and performance against abrasive wear. Protective coatings for metals, medical device adhesives, and even dental resins each benefit from IEM’s ability to form strong crosslinks. Many optical and electronic encapsulants use IEM-based chemistries to achieve weather-resistant, optically clear layers. Some waterborne and UV-cure formulations rely on IEM to generate instant curing and robust finish. The push for 3D-printable photopolymers adds another layer of relevance, with additive manufacturing shops exploring IEM-based blends for intricate, functional parts.
Academic teams tying together organic synthesis and applied polymer research have used IEM in efforts to design responsive materials—think of hydrogels that stiffen in response to temperature or pH. Major research universities focus on optimizing reaction conditions to minimize waste and lower residual monomer levels in finished products. Controlled radical polymerizations, such as Atom Transfer Radical Polymerization (ATRP) or Reversible Addition–Fragmentation chain Transfer (RAFT), help tune polymer architectures using IEM as a clickable module. Some groups explore IEM as a crosslinker in soft robotics, where flexibility must walk a fine line with resilience and chemical stability.
Long-term exposure studies on isocyanates, including IEM, raise concerns about respiratory sensitization and chronic inflammation. Animal studies show that even low-level inhalation can cause lasting airway hyper-responsiveness. The isocyanate group’s high reactivity also means that protein binding in the skin or lungs acts as a catalyst for allergies. Occupational data from factories using isocyanate intermediates suggest careful air monitoring and strict medical surveillance programs reduce the risk of acute or chronic sensitization. Ongoing studies look for methods of early detection and substitution, especially for workers who cannot avoid routine exposure. Academic publications track breakdown products in water systems, since incomplete polymerization can leave behind unreacted monomer that may leach over time.
The story of IEM is still unfolding. The move toward green chemistry has prompted calls for safer, more biodegradable analogs. Some companies invest in renewable feedstocks, experimenting with bio-based isocyanates to cut reliance on petrochemicals. New regulations in the US and Europe pressure manufacturers to lower free isocyanate levels in finished resin systems, which means those working in R&D keep searching for process tweaks and post-polymerization treatments that can lock in all reactive groups. The growing field of functional nanocomposites sees IEM in hybrid resin blends for high-value electronic, biomedical, and precision hardware, where fine-tuned crosslinking gives products distinct advantages. As 3D printing marches forward, new methods for rapid curing and post-curing of IEM-derived polymers could unlock performance gains in next-generation flexible devices or medical hardware. Industry insiders expect the push for circular economy and non-toxic ingredients will keep reshaping the development story for IEM-based materials.
Walk through a modern hospital or step into an electronics plant, and you bump into results shaped by 2-Isocyanatoethyl Methacrylate, often called IEM. This chemical steps in across industries that rely on high-quality adhesives, resins, coatings, and specialty plastics. A lot of surfaces, from dental fillings to rugged laptop shells, depend on the toughness and flexibility built into IEM-derived products.
IEM carries both a methacrylate and an isocyanate group. By blending these two chemical features, manufacturers unlock materials that bond hard plastics or metal parts together, form protective outer layers, and strengthen things like windshields and sports gear. It’s common to find IEM as a linking agent, bridging organic and inorganic surfaces. Anyone who’s ever snapped the handle off a cheap tool or watched chipped paint flake off a new car hood knows the headache of brittle or poorly-bonded materials. Scientific journals show that adding IEM into the equation creates coatings with better scratch and chemical resistance (Polymers for Advanced Technologies, 2020).
Chemistry teachers always stress the importance of crosslinkers. I remember fighting with loose ornaments in high school shop class glue projects—if only I’d had something tougher than Elmer’s. IEM stands out in high-performance adhesives. Dental labs, for example, use IEM to toughen up fillings and crowns. Studies from the Journal of Dental Research point out that methacrylates with isocyanate groups boost the durability and bonding power of dental resins. With IEM in the mix, dental work lasts longer under the wear and tear of daily eating and cleaning.
Safety looms large in handling raw isocyanates, and IEM isn’t different. In factories, workers must wear heavy-duty gloves and face shields because isocyanate vapors irritate skin and lungs. At the same time, tight rules and careful handling mean these chemicals can help build safer products down the road—by sealing lead paint behind strong coatings, for example. The European Chemicals Agency lists IEM as a substance that needs respect, not avoidance, in the workplace. I find that manufacturers who train staff thoroughly and use updated ventilation don’t just keep people safe—they also avoid downtime and lawsuits.
Construction and electronics companies face one problem over and over: keeping products strong without making them bulky or brittle. Products built with IEM-based resins stand up better to tough conditions. Laptops take more knocks. Solar panels last longer on salty coastlines. Lighter, more durable coatings save factories money, use fewer resources, and put fewer chemical scraps into the trash. Research into methacrylate-based composites, highlighted in the Journal of Materials Chemistry, keeps driving new applications, from flexible sensors to fuel-efficient vehicles.
Engineers and scientists have plenty of work ahead to push for safer substitutes or even greener methods to make high-value materials. Academic partnerships with industry spark new ways to recycle or neutralize chemical byproducts. Work on plant-based methacrylates looks promising but still needs a bigger push to match IEM’s performance. Until then, the careful use and handling of chemicals like IEM let everyday products last longer, break less often, and do their jobs more reliably for people everywhere.
2-Isocyanatoethyl Methacrylate doesn’t look dangerous at first glance, but make no mistake, this chemical packs a punch. It’s used in making coatings, adhesives, and special plastics, but its reactive nature can create hidden hazards in the workplace. Someone familiar with labs knows a little splash or accidental inhalation can cause big problems, from skin blistering to serious respiratory irritation. That’s not just chemical literature speaking — I’ve seen what happens after careless glovework or forgetting to close a bottle.
A pair of nitrile gloves can mean the difference between an annoying afternoon and a hospital visit. Avoid latex — it doesn't give enough protection here. Wear goggles that create a proper seal, not flimsy safety glasses. Long-sleeve lab coats and chemical-resistant aprons should cover any exposed skin. Shoes cover your feet, so leave the sandals at home. A good practice is always treating this stuff like it will land on you, even if you think your hands are steady.
Isocyanates don’t wait around for you to notice them. You can’t rely on your nose — these fumes start causing damage before you even sense them. Use a certified fume hood, or a local exhaust system that pulls vapors away from your face. If there’s a risk of heavy exposure or the work moves beyond a hood, switch to a fitted respirator with organic vapor cartridges. Always double-check the filter type before starting up, and make sure it fits well. No gaps.
I’ve seen folks rush to pour and mix in whatever space is open, but that’s a shortcut that costs. Work in a spot designed for chemical handling, somewhere with good ventilation and spill control equipment nearby. Keep spill kits with absorbent pads and neutralizers within reach. Wipe surfaces regularly, and keep all containers closed until needed. Store isocyanates away from moisture and amines since a surprise reaction gets out of control quickly. Label everything and train anyone in the area to know what those labels mean before anything gets opened.
Accidents happen, even with the best plans. If a spill occurs, evacuate anyone not involved in cleanup. Use appropriate personal protection before containing or neutralizing the material. For small spills, scoop it up with absorbent material and dispose of waste according to hazardous chemical guidelines. For skin contact, wash the affected area with soap and water right away, then seek medical attention. If it gets on clothing, remove those layers immediately. For eye exposure, keep the lids open and rinse under running water for at least 15 minutes. Don’t tough it out — call for help.
Chemicals like 2-Isocyanatoethyl Methacrylate remind me of the importance of building a culture of respect around hazardous materials. Regular safety training means everyone knows how to handle, store, and dispose of chemicals safely. Encourage honest communication about mistakes or near-misses, and review what went wrong. Shared knowledge keeps people thinking on their feet, not making hopeful guesses. Solid habits and a little humility keep the worst surprises at bay.
I’ve come across plenty of specialty chemicals over the years, but 2-Isocyanatoethyl Methacrylate stands out. The chemical formula is C7H9NO3. At its core, the structure packs a methacrylate group on one side and an isocyanate group dangling on the other. Digging in, the backbone runs like this: a methacrylic acid chunk, which looks like CH2=C(CH3)COO–, joins with an ethylene bridge (–CH2CH2–), ending in an isocyanate group (–N=C=O).
An image comes to mind: CH2=C(CH3)–COO–CH2–CH2–NCO. This isn’t just textbook trivia; this set-up steers its chemistry. The double bond lets it polymerize. The isocyanate tag opens doors for cross-linking, sticking to all sorts of partners in a chemical reaction. Beyond the structure, these two groups shape its uses and safety profile.
Back in college, our lab groups often fought over samples of this stuff. Combine its two reactive ends, and you get adhesives that actually last. The isocyanate group reacts fast with things like water, amines, and alcohols. Years later, I watched teams in coatings plants rely on that exact reaction to get tough, flexible finishes. Scratches stayed shallow, and raindrops beaded rather than soaking in. There’s science behind it: the isocyanate group makes strong bonds that don’t fold under stress.
No glossing over the downsides though. Mishandling isocyanates can irritate skin and lungs, even at low exposure. I got a small splash on my glove once, and even that little contact felt itchy after a few minutes. Proper gloves, goggles, and ventilation keep people safer, and good training makes the difference in handling this compound responsibly. Agencies like OSHA set limits for workplace exposure for a reason; ignoring those rules leads to real injuries.
Fact is, knowledge of the chemical’s make-up brings smarter ways to use and control it. Companies looking to make high-performance paints, medical adhesives, or specialty polymers often want exactly this mix of reactivity. Each batch checked by infrared spectroscopy or chromatography keeps purity up and byproducts down. A higher standard for raw materials helps everything downstream, from factory yields to how safe your home renovations feel a year later.
That said, handling challenges stick around. Wastewater from production carries active isocyanate fragments into the environment if disposal skips key steps. Neutralizer solutions and closed-loop systems stop environmental leaks. In my stint consulting, I saw the best results at plants investing in thorough air filtration and resin reclamation—not just because it ticked a compliance box, but because cleanup costs skyrocket if corners get cut.
People working with this chemical want cleaner tech and more robust safeguards, not just for compliance but for peace of mind. Training, up-to-date safety data sheets, and real-time workplace monitoring offer the most direct improvement. Investments in greener chemistry—think less toxic substitutes for certain applications—promise better air for everyone. Tighter process controls mean workers stay healthier and companies avoid costly downtime.
The secret’s in that formula: each atom placed with a purpose, inviting good design but only if handled with matching care.
Chatting about chemicals often feels like stepping into a different world, but in labs and factories, everyday workers face these bottled dangers in real time. 2-Isocyanatoethyl Methacrylate stands out as one such chemical. Its sharp odor and ability to irritate skin and eyes don't invite carelessness, yet anyone who’s handled it knows a simple misstep causes entire shifts to come to a halt.
Ignoring safety data sheets invites trouble. This stuff isn’t just harsh on the nose; it reacts with water, forms hazardous products with heat, and attacks unprotected hands. The story never ends well for the person who figures this out by accident, especially in shops with lax habits or outdated training posters.
Experience tells me: toss this bottle onto a cluttered shelf, and you beg for leaks or a chemical reaction. Instead, tuck it in a dedicated corrosives cabinet with a padlock. Cool, dry, and well-ventilated spaces beat a sunny windowsill any day. I’ve watched containers warp and bulge after someone stored chemicals next to radiators. Those incidents end with emergency room visits and a long conversation with the safety officer.
I once worked in a lab with concrete floors and metal cabinets designed for nasty substances like this. We bolted them down. Every cabinet had a lip at the bottom to catch spills, shelves with lining that could be swapped if something nasty soaked through, and tubes connected to exhaust vents. Air shouldn’t get trapped around this material, so engineers placed ducts high and low. The maintenance crew kept a schedule, and if the thermometer inside climbed close to room temperature, we investigated. Old hands never shrugged this off—heat ruins the shelf life and sparks trouble.
Bad containers cause most headaches. If you notice sticky residue or cracked seals, don’t touch—call in a hazardous materials pro. Fresh containers should have the manufacturing date, batch code, and warning labels visible. Try pouring this stuff with gloves: a splash means a nasty burn that lingers for weeks. Thick nitrile or neoprene gloves hold up, but I’ve seen vinyl gloves fail after two minutes.
No chemical wins against careless staff. Every person around these materials should train with sample spills, quiz sessions, and bottle inspections. An emergency shower and eye wash should sit close by. My first time handling 2-Isocyanatoethyl Methacrylate, I thought the eye wash was overkill. After a tiny splash hit my cheek and the burning started, I became a believer.
Digital tracking works better than clipboards. Logging usage, storage times, and temperature history brings accountability. Automation, from remote alarms to scheduled checks, cuts human error. Regular audits stop old habits from slipping back in.
2-Isocyanatoethyl Methacrylate doesn’t respect shortcuts. Safe storage is not about ticking boxes—it’s about real people who head home in one piece after every shift.
Working in labs and manufacturing plants, chemical names can fade into lists—until someone coughs, itches, or finds breathing gets harder. 2-Isocyanatoethyl Methacrylate catches attention in safety circles for good reason. Linked with skin and respiratory issues, exposure doesn’t just mean a minor irritation. It triggers some serious health concerns, ones that linger if left unchecked. My own years around chemical plants taught me that underestimating a volatile compound can bring regret and sick days, not just for a few, but for whole teams.
Contact with this isocyanate often starts small—itching, redness, watery eyes. Skin, easily overlooked as just an unfortunate rash, can turn into blistered patches or even full-on allergic dermatitis. The stuff gets through gloves that weren’t meant for it. Inhaling even the tiniest amount can bring tight chest, sneezing, and a wheezy cough. Asthma risk with methacrylate exposure shows up in clinical research. American Journal of Industrial Medicine reported workers facing lasting breathing trouble long after walking off their shift, in industries from adhesives to plastics.
Repeated exposure makes things worse. Sensitization sneaks up after a few encounters, leading the immune system to treat every whiff like a new threat. Colleagues who joked about “chemical colds” ended up with real asthma. According to OSHA, isocyanates belong to a class that sends workers to clinics, sometimes unable to work again. These are not just statistics; they’re people quitting jobs, fighting insurance battles, and searching for clinics that don’t pass them off as fakers.
Research in occupational medicine journals ties chronic exposure to 2-Isocyanatoethyl Methacrylate with lung inflammation. It stretches further, with the immune system stuck in overdrive, causing lifelong sensitivity to chemicals this compound resembles. For me, the sight of an asthmatic co-worker wheezing in the break room made the research real—this isn’t a risk you just walk off. Treatment isn’t simple; corticosteroids can’t erase all the damage, and antihistamines do little when lungs clench shut. Skin problems linger for months, even years, with scarring or pigment changes reminding workers what’s at stake.
No one wants to wear a hazmat suit all day, but skipping protection opens the door to preventable illness. Good ventilation, chemical fume hoods, and gloves built for the job save skin and lungs more times than any medical intervention. Leading companies train workers on real symptoms—not just textbook ones—but also teach how to handle spills instantly. I remember a team lead who always told new hires, “If you smell something weird, get out before you try to play tough guy.” That kind of advice sticks. Enforcement of strict limits, surprise air checks, and lab tests should not slip just to hit production targets. Companies see fewer compensation claims, better morale, and skilled staff who stay healthy when they get it right.
It boils down to respect—for the compound, for education, for the people who clock in and out day after day. Whether you handle chemicals or sit in offices nearby, knowing the risks means fewer regrets later. Trust facts, trust people’s stories, and insist on safe work spaces—because one exposure can change everything.
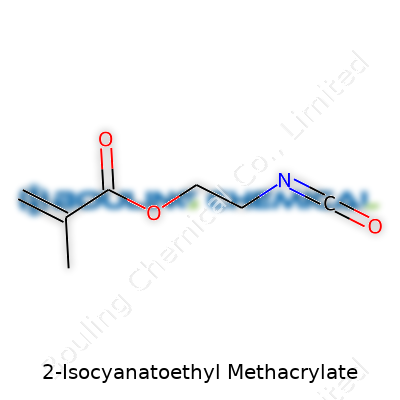
| Names | |
| Preferred IUPAC name | 2-isocyanatoethyl 2-methylprop-2-enoate |
| Other names |
2-Isocyanatoethyl methacrylate HEMA-IC Methacrylic acid 2-isocyanatoethyl ester 2-Isocyanatoethyl 2-methylprop-2-enoate Methacrylic acid, 2-isocyanatoethyl ester |
| Pronunciation | /tuː aɪˌsoʊ.saɪˈæ.nə.toʊ ˈɛθ.ɪl ˌmɛθ.əˈkræ.leɪt/ |
| Identifiers | |
| CAS Number | 3069-29-2 |
| Beilstein Reference | 1739639 |
| ChEBI | CHEBI:87164 |
| ChEMBL | CHEMBL4163287 |
| ChemSpider | 162534 |
| DrugBank | DB14007 |
| ECHA InfoCard | 03f219b5-c4a5-4e20-93e0-839b4ea2cfa3 |
| EC Number | 607-133-00-0 |
| Gmelin Reference | Gmelin Reference: 83455 |
| KEGG | C18673 |
| MeSH | D015590 |
| PubChem CID | 11224694 |
| RTECS number | UC6875000 |
| UNII | HG3ZL8W63D |
| UN number | UN3334 |
| Properties | |
| Chemical formula | C7H9NO3 |
| Molar mass | 185.18 g/mol |
| Appearance | Clear yellow to amber liquid |
| Odor | Sharp, pungent |
| Density | 1.045 g/mL at 25 °C |
| Solubility in water | Reacts with water |
| log P | 0.98 |
| Vapor pressure | 0.019 mmHg at 25 °C |
| Acidity (pKa) | 13.03 |
| Basicity (pKb) | 1.92 |
| Magnetic susceptibility (χ) | -7.53 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.454 |
| Viscosity | 20 mPa·s (20°C) |
| Dipole moment | 4.23 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 389.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS06, GHS08 |
| Pictograms | GHS02,GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H312, H314, H317, H331, H334, H335 |
| Precautionary statements | P210, P261, P264, P271, P272, P280, P284, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P333+P313, P337+P313, P362+P364, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 3-2-2-W |
| Flash point | 67 °C |
| Autoignition temperature | 225°C |
| Lethal dose or concentration | LD50 (oral, rat): 228 mg/kg |
| LD50 (median dose) | LD50 (Oral, Rat): 196 mg/kg |
| NIOSH | NIOSH: AK3759000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.005 ppm |
| IDLH (Immediate danger) | No established IDLH value. |
| Related compounds | |
| Related compounds |
2-Hydroxyethyl methacrylate Methacrylic acid Methyl methacrylate 2-Isocyanatoethyl acrylate Ethylene glycol dimethacrylate |