Back in the early decades of organic chemistry, chemists started hunting for versatile building blocks to drive research forward. Iodinated heterocycles, and specifically 2-iodothiophene, soon drew attention. Lab notes from the mid-1900s show that folks working on functionalized aromatic compounds realized the value of these iodothiophenes for forming carbon-to-carbon and carbon-to-heteroatom bonds. At that time, simple halogenation protocols often left behind messy product mixes. Over time, researchers came up with sharper and cleaner methods, and the compound started to show up in more and more reactions, making chemistry toolkit just a bit richer.
2-Iodothiophene stands as a clear example of a well-recognized specialty compound. People rely on it for its usefulness in pushing complex synthesis tasks forward, especially since the iodine atom acts as a convenient launch pad for modification. It has become something of a staple on the shelves of medicinal chemists, agrochemical developers, and electronics material researchers. Time and again, labs reach for this compound for cross-coupling efforts, targeted functionalization, and as a modular building block because its preparation and performance stay reliable.
This compound appears as a pale yellow to light brown liquid, sometimes even a low-melting solid depending on storage conditions. The scent has that usual sharp halide note, mixed with a bit of sulfur. Molecular formula C4H3IS pinpoints its makeup: a five-membered aromatic ring with a single iodine atom in the two-position, right next to sulfur. People note a melting point near 10°C and boiling somewhere in the 150–155°C range under reduced pressure. The iodine's weight and size tip the balance, making this a pretty dense compound relative to its ring structure. Its reactivity stays high, especially in transition-metal catalyzed reactions, thanks to the leaving group quality of iodide.
Bottles and vials of 2-iodothiophene in labs usually carry CAS number 928-50-7. Purity grades hover around 97% or better for most synthetic uses—higher if researchers worry about elemental contamination or trace halides. The material typically comes stabilized under nitrogen or other inert atmosphere, especially when buyers request bulk quantities. Safety labeling always warns of eye and skin irritation risk, and the chemical gets flagged as hazardous if handled carelessly or inhaled for long stretches. Labels point out the need to keep it cool, away from open flames, and sealed tight.
Most common lab routes for making 2-iodothiophene involve electrophilic iodination. Often, folks start from plain thiophene and react it with iodine in the presence of oxidants or with mixtures like iodine monochloride. Care with temperature and solvent selection makes all the difference: a too-hot flask or a too-basic solution brings out unwanted polyiodination or charring. Some prefer running the process with silver sulfate or even copper(II) sulfate as catalysts. There has also been solid progress using palladium-catalyzed direct C–H activation, which lets chemistry skip multi-step detours, but that requires more expensive setups.
In my own time with 2-iodothiophene, I have seen it serve as a real workhorse for Suzuki-Miyaura and Stille couplings, letting teams tack on new aromatic arms or functional groups onto the ring. People exploit the iodine’s willingness to leave, allowing substitution reactions that put silyl, alkynyl, or nitro groups at a precise spot on the ring. Chemists interested in fabricating conductive polymers take 2-iodothiophene as a key precursor for tailored polythiophenes, where the modifications change electrical and optical properties. Each time I see a new ligand or cross-coupled product come from this molecule, it reminds me of how critical such small but reactive units are to larger advances in technology.
If you search commercial catalogs and chemical repositories, you’ll spot several alternative labels: Thiophene, 2-iodo-; 2-Iodothiofene; and simply 2-IT. Literature from Japanese and European sources may use slightly different spellings or abbreviations, but the CAS number keeps things clear when ordering. Some suppliers sell it under their own tradenames, but most scientists stick to calling it by its systematic name or abbreviation for clarity.
Everyone who has handled 2-iodothiophene in a teaching or research lab learns to keep gloves and goggles on, and a working fume hood is non-negotiable. The compound can irritate eyes, skin, and respiratory systems. Not many people realize the environmental impact, either; iodo-organics can mess with aquatic organisms, so disposal through regular drains is out of the question. Spills call for absorption with inert material and lab waste procedures. Training for handling and first-aid is important, and I have always made sure to walk new students through the right protocols before a bottle gets opened.
Researchers purposefully reach for 2-iodothiophene in the field of pharmaceuticals and agrochemicals, especially when custom-building molecules with sulfur and heavy halogen atoms. Its flexibility for cross-coupling shines in making advanced materials—OLEDs (organic light-emitting diodes), solar cell conductors, and new-age batteries all have materials history that traces back to 2-iodothiophene somewhere along the supply chain. Medicinal chemists often use it as a stepping stone to anti-infective or anti-inflammatory compounds. I have seen synthetic groups spend weeks mapping out the next coupling modification, all starting from this modest but crucial building block.
New ideas keep popping up in the realm of iodothiophene chemistry. Green chemistry pushes for less wasteful or more sustainable synthetic routes, cutting down on toxic solvents and above all, precious heavy metals. Electrochemical and photoredox techniques grow more common. Teams focus energy on achieving selective multi-step syntheses straight from cheap starting materials. A lot of computational chemistry groups start by modeling electron distribution in the iodothiophene ring to predict and then verify with hands-on experiments how a new catalyst or functionalization might behave. Broadly speaking, the quest is to stretch how and where this compound can open new synthesis pathways.
Toxicological profiles for 2-iodothiophene, though not as thick as for some industrial solvents, do show possible skin sensitization and moderate toxicity by ingestion or inhalation. Animal studies remain sparse but suggest the iodine atom’s presence increases both reactivity and persistence in biological systems. People in industrial safety review boards review new toxicity data as part of chemical safety sheets; university and corporate labs make sure to limit acute and chronic exposure. The waste from syntheses involving iodothiophenes, if not handled wisely, risks not only human health but wider aquatic life, so it pays to follow rigorous disposal protocols.
Having spent enough time on the synthetic side of chemistry, it’s clear that 2-iodothiophene will keep making headlines in both research and industrial journals. More environmentally responsible preparations and applications are closer to reality with each new catalyst or process innovation. The steady march of electronics demands heterocycles with precisely tuned properties, so this molecule likely will anchor many of the next big leaps, from new pharmaceuticals to flexible screens. Startups and academic groups work on minimizing byproducts and cutting the cost of large-scale production. People keep searching for ways to recycle or repurpose derivatized iodothiophenes after use, either into safer degradation products or back into synthetically valuable forms, so the story of this compound looks set for plenty more chapters.
Thinking back on my days of scribbling chemical structures late at night, I remember how easy it felt to overlook the impact of a single atom swap. 2-Iodothiophene, with its ring of four carbons, a sulfur, and a bold iodine at position two, stands as an example of how science shapes up on the molecular level. The actual formula—C4H3IS—doesn’t look complicated to anybody used to organic chemistry, but behind those letters sits a small story of curiosity about the world.
It’s tempting to gloss over molecules like this, but small changes in their makeup often lead to big shifts in how they behave. Swap a hydrogen for iodine, and suddenly the molecule is primed to interact with other chemicals in novel ways. In pharmaceuticals and materials science, just a few tweaks can breed drugs with sharper effects or materials that stand up in harsher conditions. I grew up hearing about “magic bullets” in medicine—those drugs hauled out by persistent researchers who noticed odd little features in molecular formulas.
In the lab, I saw firsthand how 2-iodothiophene’s iodine attachment amplifies its value. That heavy iodine atom isn’t just decorative; it’s a strong anchor point for synthesizing more complex compounds. Researchers leverage that to build molecules tailored for electronics, solar cells, and experimental cancer drugs. Most breakthroughs, honestly, ride on such meticulous attention to molecular detail.
C4H3IS captures the skeleton of 2-iodothiophene in shorthand, but you really need to picture the atoms snapped together in a five-membered ring—a sulfur thread woven in, three hydrogens balancing out the carbon count, and iodine clinging to carbon number two. In both undergraduate and graduate labs, I learned that missing a single atom or misplacing one on a ring could send an experiment into the weeds. If the structure dropped so much as a hydrogen, an entire set of physical, chemical, and biological properties would shift.
Science education, especially in high school and college, tends to skim past compounds like 2-iodothiophene unless someone nudges you to dig deeper. In research, though, accuracy stands front and center. If a student writes C4H4SI on a test, that slips past correct, and every misstep in the formula could undermine a project. Getting the formula right isn’t about rote memorization, but about fostering a respect for detail—something all scientists and educators should pass on.
In industrial chemistry, the right molecular formula saves money and time. Synthetic chemists know that mislabeling or misreading a formula can render an entire run useless. That’s a message every young scientist should take to heart: details are the difference between progress and stalled projects.
It helps to build strong habits: double-check formulas, draw out the rings—whatever locks in the value of being precise. Even if most folks won’t ever handle a vial marked “2-iodothiophene,” the message carries over to countless other areas. Any field—lab work, engineering, even cooking—rewards that kind of focus on accuracy.
C4H3IS: short, sweet, and a reminder of how paying attention paves the way for both breakthrough science and successful everyday work.
I’ve crossed paths with a lot of tough molecules in research labs and organic syntheses, but 2-Iodothiophene always finds a way to demand a bit more respect than most. Anyone who’s handled aromatic iodides before knows certain compounds may seem stable at first glance, but a slack approach can lead to ruined batches or worse, safety scares. This ringed compound helps build pharmaceuticals and advanced materials, but if you ignore storage, costs pile up. Let’s talk straight about what it really takes to keep it in its prime.
Once you unscrew that fresh bottle, you’ll notice an unmistakable odor—reminds me of that sulfur and sea tang from undergrad days. Ignite your curiosity, but not your workspace. Like other halogenated aromatics, I’ve seen 2-Iodothiophene break down when exposed to light, air, or moisture. It loves to react with just about anything that gives it an excuse. Each time someone leaves a cap off or forgets to flush bottles with inert gas, you lose some to degradation or contamination. We’ve had brownish samples months after opening: the lesson sticks quick.
I learned from older chemists that glass vials do the heavy lifting for these sensitive compounds. I always use amber-colored ones—clear glass just lets UV rays sneak in. Sunlight kicks off decomposition faster than people expect, so a dark storage cabinet saves headaches. Moisture really eats away purity, especially if you live in a humid area. Silica gel packets keep things dry, even as you work in and out of the container.
Temperature matters more than most realize. Leaving it out at room temperature often works for a short stint, but the bottle stays at its best in a fridge. Not every fridge, either. Get a dedicated lab refrigerator. Keeping food with chemicals has ruined more lunches and experiments than I’d care to admit. Cool, but not freezing—condensation ruins the party inside the container if temperatures swing.
Oxygen exposure has its dangers. I always flush opened vials with argon or nitrogen before closing tight, especially if I know the sample’s got a long bench life ahead. Those inert gases cost money, but so does tossing out spoiled chemicals mid-project.
I used to scribble dates on masking tape, thinking I could rely on memory or sharp eyesight. You wind up confusing new stuff with old. Batch number, opening date, and even the supplier information should go on that vial. Nothing sours team spirit like hunting for who contaminated the last usable sample.
Seeing money wasted on spoiled chemicals hurts, especially for smaller labs. Labs can pool purchases, splitting high-value chemicals to lower costs. Inventory checks every month kept our stocks healthy and stopped over-ordering. Spill kits and fume hoods nearby are non-negotiable; even a minor spill turns messy if you get complacent.
For disposal, following your institution’s waste protocols keeps you on the safe side. Dosing out tiny quantities at a time, rather than pouring from a large drum, lessens accident risk and stops all your stock from degrading in one go. Training new folks pays back tenfold; passing these habits down means every chemist saves time and money in the long run.
Anyone who’s spent time in a chemistry lab has a mental list of the “go-to” building blocks for making new compounds. 2-Iodothiophene sits high on that list. I’ve seen researchers light up when they lay hands on it, and not because it’s some rare exotic—just because it keeps proving useful. With an iodine sitting on that delicate thiophene ring, this compound opens up a ton of possibilities. For chemists, it’s less about showing off and more about solving problems.
Most new medicines don’t pop into existence on their own. Behind every anti-inflammatory or antiviral, there’s a journey through big chemical roadblocks. 2-Iodothiophene acts like a trusty wrench for breaking through those stuck bolts. It steps in during cross-coupling reactions, carrying fragments and bridging them together, especially in Suzuki or Sonogashira couplings. This connection matters because new coupling reactions can mean new drug molecules, with better activity or less toxicity. Over the past decade, I’ve seen medicinal chemists expand their chemical “toolbox” by leaning on this iodine-substituted compound. They’re not just after drugs—the same reactivity helps chemists design better display materials and organic electronics, where the arrangement of atoms makes all the difference.
Plenty of academic labs use 2-Iodothiophene as their entry point into synthesizing more complex ring systems. Whenever someone sketches out those odd-shaped aromatic molecules found in sensors or organic semiconductors, the conversation lands on where they’ll get their building blocks. 2-Iodothiophene offers a reliable way to build up sulfur-containing organic frameworks. By sticking that iodine in the number two position, chemists get more control over the final product’s shape, size, and chemical activity. It’s not a matter of tradition—for a lot of researchers, it’s about reliability and options.
Ask anyone who works in organic synthesis about bottlenecks, and you’ll get an earful about cost, stability, and purity. 2-Iodothiophene isn’t immune. On more than one occasion, I’ve had to shop around for a decent bulk supplier. Pricing fluctuates with the iodine market, and purity can ruin a reaction if you cut corners. Solutions for this often mean working with suppliers who know what labs really need, or running better purification checks in-house. Folks have also started looking for greener ways to make thiophenes, because classic iodine-based chemistry can create a mess that nobody wants to clean up.
The push for new technology puts steady pressure on useful chemicals like this one. Pharma wants faster, cleaner reactions. Tech firms want lighter, more efficient conductive materials. 2-Iodothiophene keeps holding its ground because it saves time, unlocks new reactions, and pairs well with modern synthetic tricks. The next step might come from blending this classic building block with computer-aided design or greener chemistry routes. As folks in both academia and industry keep aiming higher, the demand for robust starting materials will only keep rising. 2-Iodothiophene will stick around—not because it’s glamorous, but because it gets the job done, and at the end of the day, that’s what most chemists are after.
Most people never think about chemical identifiers, but anyone who’s handled research chemicals knows how much hinges on a string of digits. For 2-Iodothiophene, that critical label is 693-50-7. This isn’t just bureaucratic shorthand. For chemists and suppliers, a CAS number guards against confusion and costly mistakes. There’s no wiggle room in science. Using the wrong molecule—say, mistaking 2-Iodothiophene for a similar structure—could send an experiment sideways or ruin a costly batch.
You won’t find 2-Iodothiophene on a grocery shelf. It’s a specialty compound, mostly seen in research labs and drug manufacturing circles. The stakes are high here. No one wants to risk a project by relying on common names, which shift with language and region. CAS numbers cut straight through the clutter. Each number keys to one and only one compound, no matter where the lab sits.
Every synthetic chemist I’ve met trusts the CAS registry more than product labels. Experience has taught me why. Human error sneaks in easily. A missing dash or a typo in a records system can have big consequences. Sourcing 2-Iodothiophene—CAS number 693-50-7—means you get what you asked for, every time. An entire project can hinge on that certainty. As someone who’s spent nights triple-checking a stockroom before a big experiment, I don’t need convincing. Without this identifier, researchers could waste weeks chasing dead ends or, worse, stumble into safety issues by mishandling unknown substances.
Many breakthrough drugs and materials draw on molecules like 2-Iodothiophene. Its sulfur and iodine twist gives it special reactivity. Chemists make use of these properties when building larger, more complex structures. But in these intricate syntheses, a single substitution throws off predictions and sends yields crashing. The CAS number forms a checkpoint—guarding every transaction, stock entry, and experiment record. It’s more than a label. It’s security for multimillion-dollar projects.
The world’s chemical trade never stops expanding. Scientists in Seoul, Mumbai, or Boston all use the CAS system. E-mails and shipping manifests move fast. Mislabeled chemical shipments delay research or even cause hazardous incidents. One slip-up can have ripple effects—delayed papers, missed patents, shelved projects. As labs keep growing, CAS numbers offer a kind of anchor—something everyone agrees on.
No system works perfectly. Sometimes you’ll find out-of-date or wrong CAS information online. Still, few tools have done more to make research safer and faster. Relying only on chemical names once cost my team a week after a supplier mixed up regioisomers. We switched to using CAS numbers in everything—orders, labels, data logs—and overnight, those headaches stopped.
The sheer amount of research depends on solid basics. CAS numbers for compounds like 2-Iodothiophene seem dull until you realize how much they keep moving. They help all of us—the student juggling glassware, the factory tech checking barrels, the manager signing off major shipments—work better and sleep easier. For 2-Iodothiophene or any rare molecule, that registry number where everything lines up is more than trivia. In the lab, it’s a lifeline.
Some lab chemicals just don’t make the headlines the way big, nasty ones do. 2-Iodothiophene is tucked away on the shelf in many university and research labs. It isn’t a household name. The trouble is, no one wants surprises with chemicals. Understanding real-life hazards often means going beyond headlines and taking a closer look at what’s in that bottle.
2-Iodothiophene isn’t some massive industrial pollutant, and you won’t see it in cleaning products. The compound serves organic synthesis—making new molecules, mainly for pharmaceutical research, materials science, and advanced electronics. In my days working with aromatic compounds, I’ve noticed folks sometimes assume small-quantity research chemicals are “safe enough” because they’re not in bulk barrels. That’s not always true, and sometimes it just means people haven’t read the full safety sheet.
The first place scientists check for handling and risks is always the SDS. With 2-Iodothiophene, you run into a set of problems: it comes with a warning about skin and eye irritation. Breathing its vapors raises red flags, too. There isn’t much research proving exactly what long-term effects might look like, but lab animals react to similar compounds with symptoms you don’t want to experience—respiratory trouble and, sometimes, allergic reactions. Small splashes can sting or burn, and the smell isn’t exactly rosewater.
In the past, younger lab workers have sometimes scoffed at gloves or eye shields, usually because they’re hurrying. 2-Iodothiophene isn’t like water. I’ve seen what happens with casual handling: rashes and even a hospital trip after someone rubbed their eyes absentmindedly. There’s also a fire factor: it catches fire more easily than many expect. Most labs with a proper fire extinguisher setup still treat this as a real hazard, not just a theoretical one.
People sometimes underestimate the risk of any “routine” chemical. Take 2-Iodothiophene as you would any reactive or potentially irritating organic—it asks for gloves, safety glasses, and a fume hood. Open-air pouring or pipetting without vented protection lets those vapors get right up your nose. Even the dust can make your next lunch break less pleasant. In a busy or shared lab, spills get messy fast. A careless hand can send a bottle tipping, leading to little clouds or pools and, worse, confusion if no one recognized what just hit the ground.
Not every lab has a fancy chemical management system, but the basics work. I recommend labeling all containers properly and keeping incompatible substances far apart. Spill kits in arm’s reach have saved lots of headaches. Regular checks for worn gloves or eye protection matter just as much. It helps to walk newer lab members through a real demonstration of proper handling and—this is often skipped—how to dispose of leftovers safely through approved waste streams.
The simple rules save fingers, eyes, and lungs: gloves, goggles, and the hood. Ignore the temptation to “just pour this one thing quickly,” because chemicals don’t care if you’re in a hurry. In my experience, respect for the risks, even in small batches, ends up saving time, money, and pain later. So, while 2-Iodothiophene won’t hit the nightly news, it still deserves attention—one careless move can turn an ordinary day into a round of first aid and paperwork.
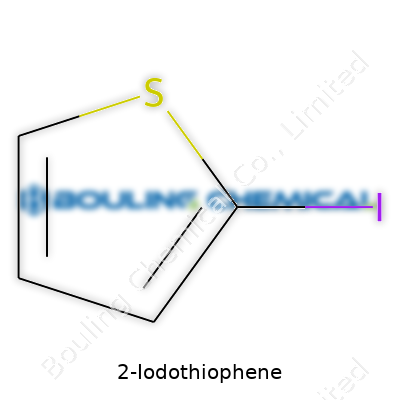
| Names | |
| Preferred IUPAC name | 2-Iodothiophene |
| Other names |
2-Iodothiophene 2-Iodothiofene Thiophene, 2-iodo- 2-Iodothiofene 2-Iodothiophen |
| Pronunciation | /tuː aɪˌoʊdoʊˈθaɪ.oʊfiːn/ |
| Identifiers | |
| CAS Number | [693-55-0] |
| Beilstein Reference | 1207553 |
| ChEBI | CHEBI:51847 |
| ChEMBL | CHEMBL501136 |
| ChemSpider | 62212 |
| DrugBank | DB08310 |
| ECHA InfoCard | ECHA InfoCard: 100.018.255 |
| EC Number | 211-880-6 |
| Gmelin Reference | 432841 |
| KEGG | C11168 |
| MeSH | D017898 |
| PubChem CID | 69780 |
| RTECS number | WN4600000 |
| UNII | 9Y6H6YY6B9 |
| UN number | UN3332 |
| CompTox Dashboard (EPA) | DTXSID10764712 |
| Properties | |
| Chemical formula | C4H3IS |
| Molar mass | 209.06 g/mol |
| Appearance | Pale yellow to brown liquid |
| Density | 1.968 g/mL at 25 °C |
| Solubility in water | insoluble |
| log P | 1.98 |
| Vapor pressure | 0.2 mmHg (20°C) |
| Acidity (pKa) | 3.74 |
| Basicity (pKb) | -4.0 |
| Magnetic susceptibility (χ) | -64.0e-6 cm³/mol |
| Refractive index (nD) | 1.688 |
| Viscosity | 1.84 cP (20°C) |
| Dipole moment | 1.96 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 162.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -4.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -219 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | Flash point: 113 °C |
| Lethal dose or concentration | LD50 (oral, rat): 2520 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 >2000 mg/kg |
| NIOSH | QJ8225000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100 ppm |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
2-Bromothiophene 2-Chlorothiophene 2-Fluorothiophene 3-Iodothiophene |