Scientists always hunt for better materials, especially ones that lend a unique edge to research and industry. Long before green chemistry became mainstream, early organic chemists tinkered with imidazole derivatives to understand how these nitrogen-containing rings could be twisted for special jobs. The story of 2-Heptadecyl-Imidazole tracks back to efforts in modifying standard imidazole by adding a long, greasy alkyl chain. This twist didn’t just appear in some academic journal; it took years of trial runs and product failures before a method surfaced that allowed the attachment of a hefty heptadecyl tail. Not every compound gets the spotlight, but this one found its own small niche for researchers aiming to tackle barriers in solubility, membrane mimicry, and even corrosion resistance. I've seen folks in the lab dig through old chemical catalogs, hoping to find a match for something like 2-Heptadecyl-Imidazole because of an offhand reference in a decades-old patent.
2-Heptadecyl-Imidazole is basically an imidazole core decked out with a C17 “handle,” which gives it a waxy, near-soapy feel and makes it feel right at home in high-end lab work exploring surfactant chemistry or advanced coatings. Chemists appreciate its capacity for forming organized films or specialized phases, especially as the need grows for smart functional materials in electronics and corrosion protection. If you examine the bottles on chemical supply shelves, this compound sometimes pops up among a group of niche building blocks, rarely sold in bulk, but chosen for jobs that standard imidazoles simply cannot do. For many in materials science, reaching for this molecule means pushing for effects beyond what short-chain imidazoles or regular surfactants deliver.
On the bench, this substance stands out as a waxy solid, off-white or yellow. Its long hydrocarbon tail drops the solubility in water close to nil, yet in nonpolar solvents, it happily dissolves. Melting usually kicks in a few degrees above room temperature—anyone warming a flask on a heat block to make it workable knows this dance well. Imidazole’s basic core remains chemically stubborn, picking up protons in acid and holding on tight to metal surfaces. Turn to the logP values (showing a high partition coefficient), and you see this molecule resists hanging out in watery environments, which actually suits loads of surfactant or membrane research.
Manufacturers and suppliers tag this compound with purity percentages, most often above 97%. Labeling focuses on the CAS number (72698-30-9), molecular formula (C24H44N2), and minor descriptions like “solid with low odor.” I've seen these jars marked in bold inks: hazard statements about irritation linger, but most folks just remember to pop on a new pair of gloves before cracking open the lid. If you’re lucky, some suppliers include small notes about storage in temperature- and moisture-controlled conditions, but it rarely stays long on the shelf in active research groups—they use what they buy, and reorder only if the project succeeds.
Building this molecule in a lab means linking a heptadecyl group to imidazole through an alkylation reaction. The recipe often uses a heptadecyl halide or heptadecanol, mixed with base and sometimes aided by heat or a dash of phase transfer catalyst. The chosen route depends on what’s safe, what reagents one can get quickly, and the scale involved. Filtering, washing, and recrystallizing the solid requires patience, given the long-chain’s sticky nature. Chemists often joke that isolation feels like wrestling a bar of soap out of a sand trap—sticky, stubborn, and demanding lots of rinsing with hexane or chloroform. Process tweaks keep coming, especially to cut down waste and boost the yield, which in turn helps keep costs in check and makes the compound a bit more accessible.
Imidazole rings love to coordinate with metals, so 2-Heptadecyl-Imidazole often features in complexation tests, especially for corrosion protection. Researchers modify this molecule by attaching other chemical groups, sometimes to the nitrogen, sometimes toggling the chain length based on specific needs—like improving compatibility with industrial coatings or tweaking the melting point for new materials. The long alkyl tail makes this molecule a strong candidate for self-assembly research or for testing the edge between soluble and insoluble amphiphiles. From personal experience, seeing these imidazole derivatives layer themselves on a metal electrode opens doors for new anti-corrosion strategies that last longer and stick better.
If you’ve spent time hunting for this molecule, you’ve probably encountered it as 2-Heptadecyl-1H-imidazole, N-Heptadecylimidazole, or even 1H-Imidazole, 2-heptadecyl-. Product labels may use code numbers or abbreviations in some catalogs, but chemists tend to rely on full chemical names or common synonyms in lab notebooks to avoid confusion—a lesson usually learned after one too many wrong bottle incidents.
Handling this chemical follows standard organic solid protocols. Even if toxicity data isn’t as detailed as for big-name industrial compounds, standard guidelines apply: use gloves, goggles, and splash-proof lab coats. Ventilation matters, especially if you’re grinding, heating, or dissolving in volatile solvents. MSDS sheets caution that the dust or vapors may cause irritation, so researchers keep spills to a minimum. Waste goes in dedicated organic bins—never mix with acids or oxidizers during disposal. Labs often run regular training for new researchers, highlighting compound-specific quirks that come with this molecule and its ilk.
The reach of 2-Heptadecyl-Imidazole stretches into corrosion protection, surfactant science, and even biomimetic membrane research. In corrosion control, scientists mix these compounds into coatings or use them as additives during electrochemical tests—results often show improved life span of metal parts. Surfactant research benefits from the odd length and functional group, which brings unique interfacial behavior, useful from detergents to emulsion stabilizers. People working on artificial membranes or vesicles appreciate a molecule that mimics the amphiphilic nature of certain biological lipids. I’ve seen patents and early pilot tests using this molecule—or its relatives—to cut costs or boost resilience in marine environments and industrial electronics.
Development efforts keep growing. Many teams chase cleaner synthetic routes and greener solvents. Right now, there’s a race to merge classic organic synthesis with bio-based starting materials, aiming to trim waste generation and energy use. Researchers always push boundaries, hoping to uncover fresh uses in advanced energy storage, anti-microbial formulations, or even selective catalysis. In university labs, synthetic challenges and exploratory projects involving long-chain imidazoles get handed down to graduate students, who bring creative tweaks to established protocols, hoping to land that elusive higher yield or patent-worthy process improvement.
Data on long-chain imidazoles remains spotty, but scientists agree that extending the alkyl chain often softens acute toxicity, though chronic effects require much more testing. Typical in vitro tests show mild irritation at high concentrations, but much of the precaution comes from experiences with similar surfactants or long-chain amines. Animal studies trickle in as regulatory bodies request more safety detail before scaling up to industrial use. For now, researchers lean on general safe handling procedures, and institutional review boards pay extra attention before approving large pilot runs.
There’s plenty of ground left to cover. Opportunities for 2-Heptadecyl-Imidazole sit at the intersection of material science, environmental technology, and smart surface design. Manufacturers look ahead to self-healing coatings, more efficient next-gen batteries, and novel anti-corrosive treatments, hoping this molecule or its derivatives will meet tomorrow’s stricter standards and growing demands. Some research teams already pivot toward bio-derived synthesis, making these long-chained molecules a bit more sustainable, and reducing the environmental footprint tied to their manufacture. Drawing from my own observations, as demands in energy and electronics grow, compounds that bridge the gap between classic organics and engineered surfactants get more attention—and 2-Heptadecyl-Imidazole stands to play a larger role in those stories as new data and applications unfold.
Few people outside chemistry departments talk about 2-Heptadecyl-Imidazole, even though you’ll run across similar names if you dive into materials science or pharmaceuticals. The catch with this chemical sits in its structure: an imidazole ring, loved by chemists for tweaking acidity and reactivity, connected to a long heptadecyl tail, giving the whole thing a greasy, “won’t-go-away-easy” character. So why does anyone care about such a compound in the first place?
This chemical works hard behind the scenes to keep metals from falling apart. It fights corrosion, which matters to anyone hoping their car chassis won’t become lace after a few salty winters, or that bridges won’t crumble. A big part of my childhood happened in the Midwest, where snow means salt, and salt means rusted pickup trucks. Manufacturers blend compounds like 2-Heptadecyl-Imidazole into coatings or lubricants because its long tail sticks to metal while the imidazole group grabs onto the surface with surprising grip. The result? Water and oxygen don’t get much of a chance to start that ugly reddish-brown reaction.
Electronics happen to be another place this odd molecule finds a home. Small connectors, circuit boards, wires—these all rely on metal, and humidity causes no end of misery in the form of corrosion and failure. I’ve tinkered with old radios and seen the “green death” of corroded copper under the covers. A thin film of a substance like 2-Heptadecyl-Imidazole blocks moisture, which means the circuit in your favorite device stands a better chance against time, sweat, and spilled drinks.
The story stretches further. Chemists look for molecules they can modify easily, making everything from pharmaceuticals to surfactants. The imidazole ring, featured here, shows up in antifungal drugs and edits to this structure produce different effects in the body or on surfaces. The long alkyl chain means the whole molecule behaves more like an oil than a powder. This quality means it can dissolve in fats, oils, and nonpolar solvents, letting it slip into products where water just won’t do the job.
No chemical comes free of questions about its long-term effects. Molecules with long alkyl chains can linger in the environment. If they wash off cars or out of buildings, they can travel through water, sometimes collecting in soil. Their persistence makes scientists twitchy because such compounds don’t break down quickly. I’ve seen this debate play out with fire retardants and stain repellents. Manufacturers, regulators, and consumers have to weigh protection against corrosion with possible side effects on water systems and wildlife.
Fortunately, chemistry keeps moving. Biodegradable alternatives attract attention. Some research groups explore plant-based imidazoles or tweaks that help these molecules break apart easier in nature. Industry demand for “green” additives grows every year. It’s the folks repairing bridges, building cars, and designing electronics who see the value of less-persistent, safer alternatives. Until change comes, though, 2-Heptadecyl-Imidazole plays its part—keeping metals intact and electronics alive, quietly holding back the march of rust and decay.
Digging into 2-Heptadecyl-Imidazole, you’ll find its weight—often called the molecular weight or molar mass—tallies up to about 374.6 g/mol. A number like this doesn't just float around in textbooks; it guides anyone running a synthesis, purifying a batch, or studying how these molecules behave. Lab folks rely on this figure every day for mixing and measuring. It never fails to impress how a simple number helps decide if the reaction goes off without a hitch or becomes a sticky mess.
The molecular formula reads as C24H44N2. There's often more to it than the sum of its atoms, though. Whenever I've weighed out a fresh bottle of this compound, trust me, things like static or even humidity cling to those decimal points. With precise numbers, chemists cut down on waste and avoid headaches that come from guessing games. Everyone gains from this clarity, including the teams pushing for greener, safer labs.
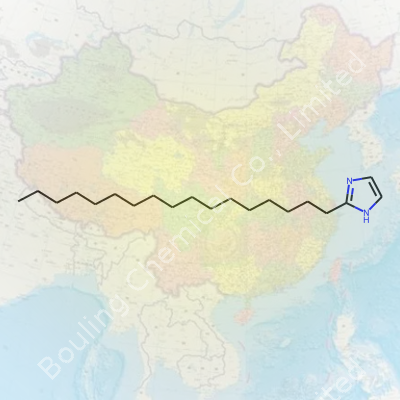
Chemically, 2-Heptadecyl-Imidazole blends the unique five-membered imidazole ring with a long hydrophobic tail. The story starts with the imidazole at its core—two nitrogen atoms at the 1 and 3 positions create a reactive, aromatic playground for scientists. At position 2 of this ring, a 17-carbon straight chain, known in the lingo as heptadecyl, stretches out. This structure, drawn out in shorthand as C7H17(Imidazole), grants the molecule unusual surface activity. In my own hands-on lab work, this combination doesn't just sit in solution politely. It gets involved, moving toward surfaces, shifting how water and oil interact, sometimes breaking up boundaries you wouldn’t expect.
Structural drawings place the ring flat, with the stringy tail leaning out. Seeing the molecule on paper always reminds me why chemistry sticks; it becomes more than theory once you find grease marks on your gloves or trace residues on glassware.
Practicality runs deep in chemical design. Detergents, coatings, corrosion inhibitors, and even certain pharmaceutical building blocks all owe their punch to a tricky balance of ring and tail. Not every molecule with a ring and a chain lands a job. By tweaking the length of that carbon chain, chemists adjust how the molecule dissolves in different liquids or finds its spot at an interface. The work I’ve seen behind the bench proves time and again: if you change up the chain by one carbon, everything from performance to safety dances to a new rhythm.
2-Heptadecyl-Imidazole stands out due to this flexibility. Its chemical backbone lets it stick to metals, block unwanted reactions, or line up in neat, consistent layers. For anyone arming themselves against rust in marine environments, or developing new surfactants with fewer toxic byproducts, knowing the molecular details saves rounds of trial and error.
Nothing goes far in chemistry without a few roadblocks. Long, greasy chains sometimes resist dissolving or spreading where you want them. Anyone who’s stirred these into cold solvents knows the frustration. Warming solutions helps, or switching to a gentler solvent, but there’s a trade-off in cost and safety.
One promising route comes from smarter molecular design on the front end. Extending research into biodegradable links or responsibly sourced heptadecyl chains could curb the environmental impact. The industry’s focus on greener chemistry isn’t window dressing; every molecule ends up somewhere—waterways, soils, or inside living things. So, anyone mapping out new uses for 2-Heptadecyl-Imidazole ought to weigh not only costs or performance but also long-term effects on the places and people sharing space with chemistry.
Storing chemicals often looks like a routine task: slap a label on a container, stick it on a shelf, job done. That routine breaks down fast with specialty chemicals like 2-Heptadecyl-Imidazole. Experience in laboratories and industry settings shows that storage isn’t simply about shelf life or regulatory compliance. The safety and usefulness of a compound hang on the way you store it. I’ve seen a lot of headaches — damaged products, surprise favorite reactions, unwanted odors, and even subtle contamination — all pointing back to slapdash storage.
This particular compound doesn’t give off dramatic warnings like some acids or oxidizers, but that’s almost worse. People might treat it like any old organic powder, forgetting what 2-Heptadecyl-Imidazole really brings to the table. Its imidazole core and long alkyl chain mean it’s neither completely inert nor wildly reactive, living in that sneaky gray area where improper storage slowly chips away at purity and consistency.
Humidity and air are some of the quiet culprits. Over time, small leaks in a cap, or an unsealed plastic bottle, will let in just enough moisture to cause the material to clump, cake, or — in bad cases — start to grow things you can’t see. You don’t want bacteria or mold anywhere near this stuff. Neither do you want airborne contaminants, which can degrade the imidazole ring or charge up the material’s reactivity in random ways.
I’ve noticed the best results come from sticking with air-tight glass containers. Plastic can be too porous, especially if you plan to store it for a few months. Some labs try amber glass, not for the aesthetics, but because some compounds just don’t like light. Even if 2-Heptadecyl-Imidazole doesn’t yellow or break down rapidly, why take the chance?
Temperature is a villain too. I’ve seen folks park their chemicals near windows or heat sources, figuring ambient lab temperature is close enough. Over weeks, a slight temperature spike can do real damage. Taking the time to use a temperature-controlled environment, like a chemical refrigerator or a climate-controlled stockroom, prevents those slow breakdowns that only show up during critical experiments.
Desiccant packets might seem old school, but tossing a fresh one in with your stored jars can make the difference between a clean compound and a useless mess. Labeling goes beyond the name and date: jot down the storage temperature, any transfer history, and even note the humidity conditions the day it was stored. Details matter. If you’re in a shared environment, communicate with your team about handling — open close the jar fast, keep it away from sinks and steamy spaces, and check seals every time.
In the end, storing 2-Heptadecyl-Imidazole shouldn’t just tick a box for compliance. Good storage habits protect investment and safety. Reliable purity means fewer ruined experiments and less downtime, and that saves time, money, and trust. The science is clear: treat this compound with brainpower, not autopilot.
Anyone poking around chemistry labs or working in specialty manufacturing sometimes runs into compounds with names that barely fit on a label. 2-Heptadecyl-Imidazole sounds like a real mouthful but boils down to a structure that grabs attention for more than just its name. It carries that imidazole group scientists recognize from biological molecules, but it’s got a big, bulky tail—a straight-chain hydrocarbon, seventeen carbons long.
Most of us remember learning at school that oil and water don’t mix. Here, the rule sticks. The long tail on 2-Heptadecyl-Imidazole acts much like the molecules making up plant wax or a slug of cooking oil. Cramming that much carbon into a molecule keeps it from mingling with water. Water prefers partners with a knack for hydrogen bonds—smaller, nimbler molecules with groups like –OH or –NH2.
I once tried to stir a little paraffin into a beaker of warm water as part of a candle-making project. No matter how long I stirred, the wax clumped and refused to dissolve. 2-Heptadecyl-Imidazole behaves as stubbornly, thanks to that long greasy chain. Strong polar solvents, especially water, just won’t do the trick.
Just because water sends this molecule packing doesn’t mean it stays out of solution in every case. Chemistry gives us plenty of options. Solvents like chloroform, toluene, and hexane welcome long-chain molecules. Hexane, in particular, offers a non-polar bath that allows 2-Heptadecyl-Imidazole to disperse and move about freely. Back in grad school, we sometimes used similar solvents to coax stubborn molecules off glassware.
You’d think alcohol, especially those with longer chains like isopropanol, might help. Yet the outcome depends on the tug between the polar imidazole head and the carbon-rich tail. Often, ethanol or methanol can dissolve small imidazoles, though they struggle once a big tail gets dragged along for the ride. Toluene, on the other hand, with its aromatic structure, matches the nonpolar qualities of that chain and opens its chemical arms wide.
Solubility isn’t just an abstract quiz question. It shapes how chemists design experiments, make pharmaceuticals, or coat surfaces. If a molecule won’t dissolve, getting it to do its job turns into a challenge. Forcing 2-Heptadecyl-Imidazole into water-based projects usually fails, unless you start fiddling with surfactants or use elaborate formulations. I’ve watched teams lose weeks fighting to blend similar compounds, only to give up and switch to oil-based systems.
On the flip side, hydrophobic chemicals like this work wonders in protecting metals from corrosion and improving coatings—industries that need water-resistant barriers. Here, the same resistance to water acts as a benefit. Solutions in toluene or other nonpolar solvents paint smooth, tough layers. Instead of fighting solubility, the approach lines up with the nature of the molecule.
Those working with 2-Heptadecyl-Imidazole end up taking cues from common sense: match the solvent to the personality of the molecule. No one expects candle wax to dissolve in lemonade, and the same rule applies here. Chemistry happens best when we understand what the molecules “want”—even if it sometimes means keeping the chemistry bench stocked with a wider array of solvents, or tweaking our expectations to play to the strengths of a seemingly tricky compound.
Anyone who’s spent time working with chemicals knows the importance of not trusting a dusty label or a vague memory. 2-Heptadecyl-Imidazole isn’t some household cleaner; it’s a specialty compound that doesn’t belong anywhere near your bare skin, open coffee mug, or yesterday’s sandwich. Each time I walk into a lab, I see where comfort turns into complacency. Gloves, goggles, and lab coats aren’t just for newcomers—they matter every shift, every batch, every experiment.
I remember the burn that comes from skipping gloves, thinking just a dab won’t hurt. Imidazole derivatives sting fast and they linger longer than you’d think. Even a small spill on your hands is no joke. Skin gets red and irritated, sometimes breaks out, and the discomfort can last all week. Protective nitrile gloves usually hold up if you check for holes, and a clean lab coat protects sleeves from soaking up a splash that ends up on your wrists or forearms. Don’t forget your eyes—goggles with side shields aren’t overkill. A coworker once lifted his glasses for a better look and spent the rest of the afternoon in the emergency wash station. One shortcut can wipe out a project and put you in the doctor’s office.
I’ve seen folks shrug off masks in a pinch, but the fumes catch up fast. Dust and vapors from 2-Heptadecyl-Imidazole aren’t just a nuisance; inhaling them brings tightness in the chest, headaches, and sometimes worse. Bringing in decent ventilation fixes a big chunk of the risk. Never mix or weigh this compound outside a fume hood or well-ventilated area—you pay the price with headaches, nausea, or worse, chronic breathing issues. Leaving windows open won’t cut it. Rely, instead, on mechanical ventilation and always check that it’s actually working, not just humming in the background.
Spills look harmless enough at first, but if left alone, the dust creeps onto desks, books, phones, and even your lunch. Wiping it with a paper towel only spreads it around. I only trust dedicated spill kits—absorbent pads, chemical-neutralizing powders, and reliable containers. Clean up fast, seal the waste in clearly labeled bags, and keep it out of reach from anyone who isn’t trained to handle hazardous material. It can feel like overkill, but it keeps the work area clean and the lab safe for everyone.
Tossing containers of 2-Heptadecyl-Imidazole onto a shelf spells disaster. I’ve seen older bottles split open or leak thanks to rough handling or stacking. Store the compound in tightly sealed containers, away from sunlight and heat sources. Choose chemical-resistant shelving, keep flammable or reactive materials far away, and always label containers clearly and indelibly. Double-check those labels before anyone borrows or returns a bottle.
Pouring leftovers down the drain is tempting when you’re short on time, but even small traces seep into pipes and the environment. Use the right hazardous waste bins, and follow the disposal guidelines set by your workplace or local authority. Disposal mistakes end up costing a whole lot more in regulatory fines and environmental damage than they save in convenience.
Most accidents happen when people feel rushed or overconfident. Real safety starts with regular, grounded training—clear, hands-on lessons make a difference even for seasoned staff. Encourage a culture where double-checking safety protocols is never seen as a lack of experience, but just the responsible thing to do.
| Names | |
| Preferred IUPAC name | 1-(Heptadecyl)-1H-imidazole |
| Other names |
1-Heptadecylimidazole 2-Heptadecyl-1H-imidazole |
| Pronunciation | /ˌtuː hɛp.təˈdɛsɪl ɪˈmɪdəˌzɒl/ |
| Identifiers | |
| CAS Number | 1120-71-4 |
| Beilstein Reference | 1697938 |
| ChEBI | CHEBI:78405 |
| ChEMBL | CHEMBL2106657 |
| ChemSpider | 21543968 |
| DrugBank | DB08983 |
| ECHA InfoCard | echa-info-card-100.231.124 |
| EC Number | 700-919-0 |
| Gmelin Reference | 83732 |
| KEGG | C09678 |
| MeSH | D017564 |
| PubChem CID | 102118171 |
| RTECS number | UP6830000 |
| UNII | A67PS5C40Q |
| UN number | UN3272 |
| CompTox Dashboard (EPA) | DTXSID0073952 |
| Properties | |
| Chemical formula | C20H39N2 |
| Molar mass | 393.39 g/mol |
| Appearance | White to almost white crystalline powder |
| Odor | odourless |
| Density | 0.9 g/cm3 |
| Solubility in water | insoluble |
| log P | 4.7 |
| Vapor pressure | 0.0000137 mmHg at 25°C |
| Acidity (pKa) | pKa = 14.6 |
| Basicity (pKb) | 4.85 |
| Magnetic susceptibility (χ) | -73.52×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.522 |
| Viscosity | Viscosity: 120.8 cP |
| Dipole moment | 2.06 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 676.7 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | -93.13 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -10761.8 kJ/mol |
| Pharmacology | |
| ATC code | D01AC19 |
| Hazards | |
| GHS labelling | GHS07, GHS08 |
| Pictograms | C1=CN=CN1CCCCCCCCCCCCCCCCC |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P273, P280, P302+P352, P305+P351+P338, P362+P364 |
| Flash point | 145 °C |
| Autoignition temperature | 240 °C |
| LD50 (median dose) | LD50 (median dose): Rat oral >2,000 mg/kg |
| NIOSH | NL0000000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 200-500 mg |
| Related compounds | |
| Related compounds |
Hexadecylimidazole Octadecylimidazole 2-Undecyl-imidazole 1-Heptadecyl-1H-imidazole 2-Heptadecyl-benzimidazole |