Looking back, the story of 2-formylpyrrole starts inside the laboratories of early 20th-century chemists who hunted for ways to tweak the pyrrole ring. Back then, scientists tried to understand why small chemical changes mattered so much in organic reactions. The formyl group linked to the pyrrole backbone delivered both new challenges and fresh promise. In those days, search for heterocyclic aldehydes meant slow filtration and glassware lined with exotic stains. At universities, folks like Robinson and Barger scraped data together, showing how simple formylation could open doors for drug design and dyes. The path to modern synthesis wasn’t straight or easy, but the curiosity behind each failed and successful trial built today’s knowledge.
2-Formylpyrrole, known to a few as pyrrole-2-carboxaldehyde, belongs to a niche crowd of aromatic heterocyclic aldehydes. In labs and catalogues, it shows up as a faintly yellow liquid or sometimes a chunky, crystalline solid, depending on storage. On its own, the molecule bridges basic research and real commercial value. Specialty chemical houses supply it for researchers who demand solid reliability in purity and reactivity, because batches gone wrong spoil months of experiments. Not a bestseller at hardware shops, but for those who recognize its shape, this compound is a staple that quietly helps build bigger molecules.
Every researcher who’s handled 2-formylpyrrole knows it by its distinctive smell—strong, a little spicy, and hard to describe. Its melting point sticks around 35 °C, and its boiling point hovers near 140 °C (at reduced pressure, or things turn messy). In water, it resists mixing; in alcohol or ether, it goes right in. Chemically, the aldehyde sits directly attached to the pyrrole ring’s 2-position, making it far more reactive than many other pyrrole derivatives. It oxidizes quite easily with strong oxidizers, and often darkens in air if left on a bench for too long. Light and heat can nudge it toward unwanted reactions, so users keep it cold or tucked away in amber bottles.
A reliable supplier will list 2-formylpyrrole’s CAS number (1003-29-8), a molecular formula of C5H5NO, and purity commonly better than 97%. You’ll spot the deep black product number, batch details, expiry, and proper hazard icons screaming “Irritant” in familiar GHS shapes. Good labels clarify storage—cool, dry, ventilated—and shipping details, because temperature shifts risk polymerization or decay. Purest stocks often pass NMR and GC-MS testing, ensuring no lingering acidity or side products, because even tiny impurities can foul up fine organic syntheses or analytical work.
The classic recipe for 2-formylpyrrole starts from plain pyrrole and a chemical called Vilsmeier reagent, made fresh on the spot from POCl3 and DMF. In a well-ventilated fume hood, pyrrole and the reagent meet and react at low temperature. Work-up involves pouring into ice-water then careful neutralization. Extraction strains the aldehyde from washing and filtration. Industrial methods may swap in chromium trioxide or manganese dioxide—strong oxidizers with their own risks and waste issues, but handy for big batches. Careful work avoids over-formylation and keeps side reactions low, since pure product matters more than quick bulk production for most uses.
Chemists rarely let 2-formylpyrrole stand alone. That aldehyde tag calls for action, and the molecule reacts with amines, hydrazines, and other nucleophiles forming Schiff bases or hydrazones. It steps easily into condensation reactions—named after scientists like Knoevenagel and Mannich—yielding sturdy frameworks for new drug candidates or electronic materials. The aldehyde’s reactivity offers countless hooks for cross-coupling, cyclization, and ligand formation. Even when used as a starting block, modifications can decorate the ring, insert new atoms, or grow long chains, tailoring function for every creative project on the bench.
Besides the main name, the catalogues throw out terms such as pyrrole-2-carboxaldehyde, 1H-pyrrole-2-carbaldehyde, and 2-formyl-1H-pyrrole. In trade lists or import logs, it can turn up as pyrrole-2-aldehyde. These aliases solve paperwork confusion and help researchers find the right material. Each label points to the same backbone, making it easier for global users to cross-check between languages or supplier codes.
Everyone handling 2-formylpyrrole should wear gloves and goggles. Skin contact may sting, eyes water quickly, and vapors chase out all but the determined. Spills demand quick action—ventilation, absorbents, and scrupulous cleaning. Storage stays below room temperature, with containers tight and clearly labeled. Waste heads for hazardous disposal, not the drain. Labs keep emergency showers nearby and MSDS sheets against the wall, because even cautious hands slip sometimes. Routine monitoring with peroxide test strips and air sniffers keeps work safe for the long haul.
Most applications for this compound pop up far from casual discussion. Medicinal chemists prize the reactivity in designing antifungal agents, CNS-active molecules, or imaging probes. Coordination chemists use it to stitch together metal complexes for catalysis or sensing. Dye makers appreciate the aldehyde for fine-tuning color or stickiness on fabric. In the world of electroactive polymers and organic electronics, the molecule crops up in precursors for new materials. Biochemists grab it for building pyrrole-containing peptides—mimicking the complicated business of natural proteins.
Research always seeks new reactions and roles for 2-formylpyrrole. Over the decades, teams worldwide have mined it for the next lead molecule, searching for stronger drugs or smarter materials. High-throughput screens run automated reactions by the hundred, hoping a tweak to the pyrrole ring brings unexpected properties. Universities report dozens of patents each year around ring-modified analogs. Many current developments target sensor arrays, solar cells, or next-generation antibiotics—fields where customization matters as much as cost per gram. Some labs push green chemistry to trim solvents and toxic byproducts in its preparation, driven by both regulation and conscience.
Toxicology data on 2-formylpyrrole lags behind that of bulk industrial chemicals, but work so far flags potential dangers by inhalation and skin contact. Animal studies hint at weak mutagenic properties, likely from reactive aldehyde moieties. Nobody publishes detailed chronic exposure results, but the similarities with other aromatic aldehydes prompt most labs to adopt a cautious approach. Repeated environmental release risks aquatic species, so effluent treatment systems intercept waste streams. Safety protocols get tighter where workers repeat exposure daily, and updated regulatory findings chase new discoveries closely.
Emerging interest in advanced materials and medicinal chemistry makes 2-formylpyrrole more relevant each year. Its flexibility suits the next wave of organic LEDs, flexible batteries, or antimicrobial coatings. Startups scan patent clusters, searching for routes to cheaper, greener, or safer production. Academic teams keep probing odd positions on the ring, hunting for smart sensors or elusive enzyme targets. The compound’s modest cost and rich reactivity let researchers dream big and fail often, betting that the next pathway could spark a leap in some forgotten corner of chemistry. For chemists willing to respect its risks and rewards, 2-formylpyrrole stands ready to prove itself in old and new applications yet to be fully explored.
Thinking about chemistry often feels like looking into a secret world. Take 2-Formylpyrrole, also known as pyrrole-2-carboxaldehyde. This compound stands out in a crowd of organic molecules, mostly because of what’s packed into its tiny structure. Its formula, C5H5NO, might not catch your eye at first glance, but behind those letters sits a small ring and a functional group that both chemists and folks in industry have come to appreciate.
Picture a five-sided ring. Four sides are carbon atoms, one is a nitrogen atom. That’s the backbone, called a pyrrole. Each hydrogen is tucked in, holding the ring together. On the second spot around the ring, an aldehyde group (–CHO) hangs off. That’s what makes it “2-formyl”—the aldehyde got attached on the second carbon, counting next to the nitrogen. Rings like this don’t just hold their atoms in place, they let them interact in ways that matter a lot for flavor, scent, and even in biotech applications.
Nature and science work together in 2-Formylpyrrole’s structure. The nitrogen in the ring brings a basic property, lending a little electron density, while that aldehyde group behaves differently, always looking for a partner in reactions. It’s that kind of difference in how those groups act that gives 2-Formylpyrrole its role in things like the Maillard reaction—the browning that brings flavor to seared steaks and baked bread.
Spending time in a kitchen makes it clear: flavor changes, and sometimes those changes start with tiny molecules. 2-Formylpyrrole pops up in cooked food, roasted coffee, and even tobacco. Its chemical structure lets it escape from heated surfaces and drift into your nose, bringing an unmistakable aroma. That might not seem like a bit deal, but the food and beverage industry depends on these compounds to recreate scents that customers crave. A cup of coffee wouldn’t have that roasty, nutty scent without it.
In the lab, I’ve seen chemists use 2-Formylpyrrole as a tool for building more complicated molecules, especially in pharmaceutical projects. Its reactivity makes it a candidate for building heterocycles—those ring structures found in antibiotics, dyes, and other useful chemicals. Missteps can ruin a reaction, so knowing which atom does what in its structure helps experts pick the right recipe each time.
Working around 2-Formylpyrrole isn’t always easy. It’s sensitive to air and light, and it can spoil if left out too long. This makes storage and handling more about care than brute strength. Labs often have to chill it or shield it from light, which adds cost and effort.
There’s no shortage of opportunities to improve things here. New storage containers could make a real difference, and advances in green chemistry promise ways to make and use compounds like this without so much waste or risk. Chemists are always hunting for milder conditions, or tweaks to the molecule’s structure, to get similar results with less fuss.
Chemistry boils down to structures, and 2-Formylpyrrole’s ring with its aldehyde side-kick does more than sit on a shelf. It drives flavor, stirs curiosity in the lab, and hints at bigger things to come as researchers build new tools for industries that touch all our lives.
It’s easy to skip over names like 2-Formylpyrrole. Most folks never bump into it unless they’re tangled up in lab work or flipping through a chemistry catalog. I remember the first time I heard the name, I was helping a friend prep for a university research project. At first, it looked like any old organic compound, but soon enough, it turned out to be a real workhorse behind the scenes in a lot of different industries.
Walk into a bakery or stroll through a coffee aisle. The sweet, nutty smell hanging in the air often comes from molecules built around pyrroles, and 2-Formylpyrrole sits high on that list. Food scientists love it. They blend it in tiny doses to mimic roasted, toasted, or caramel-like notes. For anyone who works in product development, this compound acts as a shortcut to the cozy smells and flavors people crave in snacks or baked goods. Its footprint stretches from flavoring in instant coffee to boosting aromas in cocoa-based treats.
I’ve known a few folks in medicinal chemistry, and for them, 2-Formylpyrrole is more than an obscure ingredient. It’s a building block. Many drugs and bioactive molecules, especially those aiming at the central nervous system or antimicrobial activities, trace part of their roots to this structure. The formyl group tacked onto the pyrrole ring makes it reactive, so researchers use it to craft new experimental drugs or try out different combinations, hoping to catch something game-changing. Pyrrole-based drugs have already reshaped several fields, including pain management and treatment for infectious diseases.
I once sat through a seminar where a young scientist raved about new organic materials for electronics. She talked about conductive polymers and pigments, and sure enough, pyrroles were everywhere. 2-Formylpyrrole lands in this research because it helps chemists stitch together bigger molecules with unique properties. Think display screens, solar panels, or special inks for cutting-edge circuits. Its ability to underpin new molecules with special characteristics keeps researchers coming back to it, especially those looking to push the envelope in electronics and renewable energy.
The synthetic routes rely on this compound for building bigger, more complicated chemicals. Chemistry students run into it during training. It’s handy for teaching because it reacts cleanly. Scientists tweak its structure and see what happens, leading to a better grip on reaction mechanisms. Over the years, it’s played a role in countless academic projects, with more than a few undergrad and grad students gaining experience through experiments that start with measuring out this fragrant, light yellow liquid.
The fact remains: from my own time around university chemistry clubs and industry labs, 2-Formylpyrrole stands out both for what it does now and for what it could unlock down the road. Instead of just being a niche product, it’s found a way to work itself into various corners of daily life, research, and manufacturing. The smart approach stays grounded in safety and responsible sourcing, all while supporting the next breakthrough in food science, medicine, or technology. More industries ought to pay attention to this unassuming compound and maybe look for new ways to put its unique punch to good use.
Anyone who’s spent time around chemicals knows some bottles demand more respect than others. 2-Formylpyrrole belongs near the front of that line. Its strong odor can punch through a storeroom, plus it stains skin and surfaces in record time. While it’s not the loudest chemical in a safety class, it packs enough punch to cause trouble—start with irritated eyes, end with a ruined lab bench if people get it wrong.
Many overlook where they toss their reagents, but that’s where hazards grow. A shelf right under a window or next to a heat source? Not smart. 2-Formylpyrrole breaks down faster when it catches extra heat, so keep it cool and out of sunlight. I always look for the lowest, least trafficked shelf in the flammable cabinet for this one. Temperatures near 4°C keep its shelf life from slipping away.
Glass always beats plastic for this compound. Plastic containers let molecules sneak in and out, leaching smells and sometimes even breaking down. A tightly sealed, amber glass bottle blocks both air and light—trust me, you want both. Replace the cap as soon as you finish pouring. A leaky container means your workspace will soon reek, and nobody forgets that smell.
In a crowded lab, it’s tempting to grab whatever pipette or spatula’s within reach. With 2-Formylpyrrole, that becomes an expensive shortcut. It latches onto surfaces, and contaminated tools spread headaches through entire projects. Label everything, dedicate utensils, and check the bottle before each use—murky liquid or a crusty cap means the batch met air or water and now belongs in the waste bin.
I learned early: gloves, goggles, and a lab coat aren’t negotiable for 2-Formylpyrrole. Even if you’re in a hurry, putting on proper protection saves skin from nasty stains and burning sensations. Always work within a fume hood, never out in the open, breathing easier knowing vapors go up the stack, not into your lungs.
Accidents happen, no matter how many years you have under your belt. For this compound, mop-ups start with a dedicated chemical absorbent—paper towels only make the stain larger. After soaking it all up, scoop everything into a sealed bag. Don’t send it down the drain, since environmental agencies take the release of aromatic aldehydes seriously. Call the chemical waste contractor, keep documentation handy, and avoid a fine or worse: water contamination.
Too many labs let little-used bottles sit for years, but expired 2-Formylpyrrole isn’t just unreliable—it can get unstable. Log the open date every time, and set reminders to check old stock every few months. Old, brown, viscous fluid means a wasted experiment and a possible mess to clean. Racing through a project with expired chemical just leads to headaches and repeated trials.
Chemists rarely talk up the importance of storage and handling until a bad day proves the point. Respect for compounds like 2-Formylpyrrole keeps projects moving and colleagues healthy. With the right bottle, good labels, and steady routines, this aromatic building block stays an asset instead of a hazard. Everyone in the lab—students, postdocs, even the seasoned PI—benefits when safe habits run as deep as scientific curiosity.
Anyone who's done lab work knows how frustrating contaminants can get. Even a little impurity can throw off results, cost extra time, and burn up the budget. In the case of 2-Formylpyrrole, plenty of buyers want to be sure the product lines up with what the paperwork claims, especially since this compound goes into all sorts of reactions—pharmaceutical precursors, functional materials, flavors—the list goes on.
I’ve worked with several vendors who offered the same compound on paper, but it’s always purity that separates a genuine reagent from a headache. Take 2-Formylpyrrole; if a batch only hits 95% purity, those other bits—moisture, residual solvents, trace organics—will show up as strange peaks or ghost bands during analysis. Tracking down these issues with NMR or GC-MS eats into research time.
Most researchers and scale-up buyers I've spoken to look for 98% purity as the absolute minimum. Higher-end applications, like medicinal chemistry, sometimes reach for 99% or more. Pushing for these numbers reduces uncertainty and eliminates most by-product noise that can muddle downstream chemistry. In one lab, we once traced a failed reaction all the way back to a supplier using an outdated purification method.
Before anyone commits money, purity details should come up front, not in fine print. Customers want to see clear numbers: is the material 98%, is it 99.5%? Which testing methods back up those numbers—HPLC, GC, or titrations? If the seller can’t provide a detailed Certificate of Analysis, it’s a red flag. Documentation should break down what the remaining percentage actually is—whether that’s water, another pyrrole, or just leftover solvent.
It’s tempting to go for a cheaper option if the source claims pharmaceutical-grade 2-Formylpyrrole. Without proper QC, batches can vary, and each new container might yield a new surprise. For instance, even 1% of an unexpected side product can kill a synthesis pathway or force teams to repeat purifications. In my experience, this frustrates chemists more than it helps bottom lines, leading to more wasted material than savings.
Quality control experts suggest always checking the manufacturing process. If the product comes from a synthetic route that favors a certain impurity profile, buyers should be aware of it, not left guessing. Chemical suppliers who publish their testing procedures upfront, include chromatograms, and let customers review batch data build trust and reduce problems later.
Buyers gain more confidence with transparency. A reputable offer for 2-Formylpyrrole shows not only purity percentage, but also exact figures for main impurities and testing histories for the batch. Reputable sellers respond quickly with extra QC information and don’t dodge specific technical questions. For specialty compounds like this, a conversation about supply chain, storage conditions, and analytical backup does more than a basic product spec sheet ever could.
In my view, whether you’re a small independent lab or a bigger player in the market, respecting this process saves time. A clear rundown of how pure 2-Formylpyrrole is—right from the first inquiry—sets the tone for a reliable partnership and smoother chemistry work. The best suppliers know this and make that level of clarity their standard, not a paid upgrade.
Anyone dealing with chemicals in a lab or manufacturing space learns quickly: every compound has a story, and plenty have warnings. 2-Formylpyrrole is no exception. Known in some circles as pyrrole-2-carboxaldehyde, this compound crops up in synthesis labs, research benches, and sometimes, flavor chemistry. The moral? Even small bottles deserve respect.
Start with physical properties—2-Formylpyrrole is a yellow to brown liquid, with a strong, peculiar smell that can cue even an average nose that something potent is in the air. It doesn’t take much to realize, after a spill or two, that gloves matter and good ventilation pays off.
Nobody wants surprises. The main documented risks with 2-Formylpyrrole stem from its potential to irritate. If it touches skin, people can end up with a rash, itch, or redness. Eyes fare no better—splashing a little leads to burning and tears. The real headache comes from inhalation: vapors can irritate the nose, throat, and lungs, and breathing too much can bring on coughing or a sore throat.
These aren’t rare, worst-case reports from far-off places—they’re everyday risks. The United Nations’ GHS system labels 2-Formylpyrrole as harmful if swallowed. Ingesting the chemical could lead to nausea, headaches, or in some cases vomiting. Some lab professionals compare it to the bite of mild formaldehyde, which means caution carries real value.
Vapors from 2-Formylpyrrole won’t light up as easily as gasoline, but flammability still deserves respect. Heating the substance, or working near ignition sources like hot plates or open flames, can spark fires. Firefighters responding to chemical blazes often reference the flashpoint, and for this compound, the bar sits reasonably low. Keeping storage areas cool, dry, and with good airflow helps minimize accidents.
No story about this chemical can avoid the headache of long-term or chronic exposure. Nobody has uncovered serious health effects from 2-Formylpyrrole that resemble the damage of benzene or chloroform, but those who handle it daily sometimes report nagging irritation. There’s not much evidence for cancer risk or DNA damage tied to this compound, but “not much” doesn’t mean “none.” The rule I grew up with in labs? Gloves, goggles, and never cut corners.
Most people can avoid negative outcomes by sticking to good practices—think fume hoods, nitrile gloves, and face shields for splashy work. Spills happen, so absorbents and clean-up protocols keep small mistakes from spiraling into big ones. Training on chemicals isn’t just legalese—it actually keeps people healthy. Waste disposal rules deserve attention, too; 2-Formylpyrrole shouldn’t pour down a drain or end up in open trash.
Emergency eyewash stations and showers may sound like overkill until someone in the next bay splashes their wrist and needs fast help. Without regular equipment checks, these safety nets gather dust and do little when push comes to shove.
Reliable references like the Sigma-Aldrich SDS or the European Chemicals Agency database share key details–from storage warnings to emergency first aid. Pulling up that sheet before working with a new bottle of 2-Formylpyrrole can prevent a world of hassle later. My own rule is: if a label or sheet looks faded or missing, don’t trust the bottle.
As new compounds enter labs each year, 2-Formylpyrrole probably won’t top any “most dangerous” list, but treating it with casual disregard sets up trouble. Following clear safety routines and trusting the shared wisdom of chemists does more than check boxes—it keeps people healthy, lab benches organized, and days free from regrettable incidents.
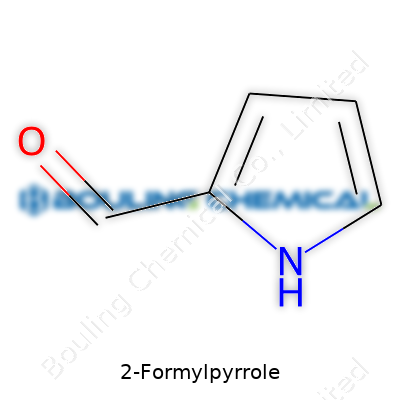
| Names | |
| Preferred IUPAC name | 1H-pyrrole-2-carbaldehyde |
| Other names |
2-Pyrrolecarboxaldehyde 2-Pyrrolylcarboxaldehyde 2-Formyl-1H-pyrrole 2-Pyrrolealdehyde |
| Pronunciation | /tuː-ˈfɔːrmɪl-pɪˌroʊl/ |
| Identifiers | |
| CAS Number | 1072-85-1 |
| 3D model (JSmol) | `/@4.12.3/3D/JSmol?query=C1=CN=C(C=1)C=O` |
| Beilstein Reference | 1209243 |
| ChEBI | CHEBI:34632 |
| ChEMBL | CHEMBL514907 |
| ChemSpider | 69957 |
| DrugBank | DB03813 |
| ECHA InfoCard | 100.014.257 |
| EC Number | 211-217-0 |
| Gmelin Reference | 63936 |
| KEGG | C01745 |
| MeSH | D017924 |
| PubChem CID | 12413 |
| RTECS number | UY8925000 |
| UNII | Z6H7H3K35F |
| UN number | UN3432 |
| Properties | |
| Chemical formula | C5H5NO |
| Molar mass | 95.10 g/mol |
| Appearance | Pale yellow to brown liquid |
| Odor | disagreeable |
| Density | 1.145 g/mL at 25 °C |
| Solubility in water | slightly soluble |
| log P | 0.23 |
| Vapor pressure | 0.0292 mmHg (25°C) |
| Acidity (pKa) | 15.5 |
| Basicity (pKb) | 11.15 |
| Magnetic susceptibility (χ) | -37.0e-6 cm³/mol |
| Refractive index (nD) | 1.5670 |
| Viscosity | 43 cP (25°C) |
| Dipole moment | 2.41 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 126.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 104.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2823 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | OC= C1=CC=CN1 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-Formylpyrrole: 2-3-0 |
| Flash point | 91°C |
| Autoignition temperature | 185 °C |
| Explosive limits | Lower: 1.6%, Upper: 12.6% |
| Lethal dose or concentration | LD50 oral rat 500 mg/kg |
| LD50 (median dose) | LD50 (median dose): 640 mg/kg (rat, oral) |
| NIOSH | WA3575000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 5°C |
| Related compounds | |
| Related compounds |
Pyrrole Pyrrole-2-carboxylic acid Pyrrole-2-carboxaldehyde oxime |