Back in the early twentieth century, labs across Europe and the United States started to notice a rising demand for new heterocyclic compounds. Chemists felt drawn to the imidazole ring for more than its challenging synthesis—its chemical reactivity and biological properties caught their imagination. The aldehyde derivative, 2-formylimidazole (sometimes known in trade as 2-imidazolecarboxaldehyde or Imidazole-2-carbaldehyde), emerged when researchers began fusing simple starting materials like glyoxal with ammonia. Academic curiosity quickly grew into utility as pharmaceuticals, dyes, and catalyst research all circled back to this compound. Synthetic routes improved over time, especially after the Second World War, as companies wanted not just small batches but scalable processes. One can see the progress in patent records and process optimization journals lining library shelves from the 1950s onwards.
2-Formylimidazole stands as a pale yellow, sometimes off-white, crystalline solid or powder. Chemical suppliers sell it in various volumes, from gram-size glass vials used by research labs to multi-kilogram drums shipped to industrial plants. It's prized for the reactive "formyl" or aldehyde group attached to a five-membered imidazole ring—a combination that invites all sorts of chemical modifications. Across catalogs, you find it under several synonyms: 2-imidazolecarboxaldehyde, 1H-imidazole-2-carbaldehyde, and simply Im2CHO. Researchers and R&D buyers know to check for these names when scanning technical data sheets.
At room temperature, 2-formylimidazole keeps to itself as a fine crystalline powder. Literature and supplier data fix its melting point between 191°C and 195°C, which speaks to its purity and consistent ring structure. Solubility profiles catch attention: it dissolves with ease in hot water and polar solvents like DMSO and methanol. Sturdy against ordinary air and light, this compound's aldehyde group still likes to react when given a nudge. Its molecular formula, C4H4N2O, comes with a molar mass of 96.09 g/mol.
Lab-grade and industrial batches follow tight quality guidelines. Labels spell out at least 97% purity—sometimes up to 99% for pharmaceutical or analytical use. Impurities, if present, usually show up on COAs as related imidazole derivatives or minor residual starting materials. Material is packed in HDPE or amber glass containers, depending on light sensitivity concerns for long-term storage. All labels must flag hazard statements mandated by international rules: H302 (harmful if swallowed), H315 (irritant to skin), and sometimes the less common H319 (irritant to eyes). Storage advisories urge cool, dry conditions, with all local environmental and safety protocols in mind.
Old school chemists used to mix glyoxal, ammonia, and formaldehyde in water, then let the solution do its work at slightly raised temperatures. This multistep reaction runs through an intermediate like 2-hydroxyimidazole before finally yielding the target compound, often followed by vacuum filtration and carefully controlled recrystallization. Experienced process chemists prefer starting from imidazole directly, adding Vilsmeier reagents such as DMF with POCl3 under controlled low temperatures, then quenching and neutralizing to avoid side-product build-up. The literature documents tweaks to solvents, temperature, and even base additives—all in pursuit of better yields, easier purification, and less environmental waste.
This aldehyde stands out in the synthetic chemist’s toolbox. Nucleophilic addition lets one create imidazole-based alcohols or imines, depending on the choice of reactant. Shiff base formation with primary amines opens the door to libraries of biologically active molecules. Cyclization reactions attract attention in medicinal chemistry circles because they let researchers expand the imidazole ring into fused bicyclic scaffolds. Students learning organic transformations can run through oxidation to imidazole-2-carboxylic acid—an easy way to demonstrate basic aldehyde chemistry, but the professionals use it to build complex catalysts and ligands for metals in specialty catalysis.
A glance at chemical registries and supply catalogs shows how many names cluster around the same compound. Chemists order it under “2-formylimidazole”, “imidazole-2-carbaldehyde”, “1H-imidazole-2-carbaldehyde”, and in some regions, “Imidazole-2-aldehyde”. The CAS number (670-96-2) brings clarity amid this pile of synonyms. Trade documentation and MSDSs sometimes abbreviate to Im2CHO or I2CA, but every formulation arrives back at the same core heterocycle and its reactive aldehyde functional group.
Handling this compound calls for respect. The pungent nature of the aldehyde group irritates mucous membranes—the reason many long-timers wear nitrile gloves and work behind fume hoods. Spills usually get cleaned up with plenty of water and proper chemical spill kits, not paper towels or improvisation. Safety data sheets list eye, skin, and respiratory irritation as real risks. In case of contact, protocols push for thorough water rinses and prompt medical checks. Standard waste protocols require collection in labeled solvent containers, avoiding any mixing with strong oxidizers or acids, and storage away from work benches open to high foot traffic. Facility managers and lab supervisors reinforce these standards through regular training, not just paperwork.
Few compounds move so easily between industries. In pharma, researchers like its role as a key precursor for antifungals and anticancer drugs. Analytical chemistry teams apply it when designing sensors for trace metals or ions. Companies focused on materials science use it in the creation of metal-organic frameworks (MOFs) and coordinated polymers—substances critical to separation science or targeted drug delivery. Dyers and pigment manufacturers occasionally build colorants on its robust ring system, drawing on stability and the ease of further modification. The breadth of uses rests on that hefty combination of reactivity, selectivity, and ease of handling.
Publications in journals such as the Journal of Organic Chemistry and European Journal of Medicinal Chemistry regularly outline new methods for expanding the synthetic applications of 2-formylimidazole. Universities push boundaries with greener synthetic pathways, looking to cut down on heavy metals and harsh reagents. Companies focused on clean tech eye catalytic cycles, using ligands derived from this compound to speed up transformations in drug synthesis or environmental remediation. Government funding sometimes supports projects that use it to create advanced polymers or sensor arrays for environmental monitoring. At every step, teams track reaction efficiency, by-product profile, and cost per mole.
Toxicologists run tests on animal models and cultured cell lines to get clarity on potential hazards. Acute exposure leads to irritation, not only because of its aldehyde function but also due to breakdown products that emerge in biological conditions. Oral LD50 levels in rodents set guidance for occupational limits in factories and pilot plants. Some early studies hint at mild mutagenicity at high doses, prompting more routine genotoxicity screening. Research usually points to rapid metabolic clearance in mammals, but any long-term contact—especially in industrial settings—gets flagged for monitoring. These data shape user handling policies and drive the design of safer analogs.
Ongoing work explores more pushbutton synthesis methods—think automation and flow reactors—for scale-up without enormous solvent waste. AI-driven predictive modeling has started to play a role: researchers input the structure and use algorithms to map out new transformations using this compound as a platform. Sustainable chemistry labs view it as a test case for switching over to renewable starting materials. From drug lead discovery to solid-state material development, 2-formylimidazole keeps drawing attention, offering the kind of reactivity and adaptability that helps bridge the gap between academic innovation and real-world products. Demand looks set to continue, as more disciplines recognize the value in reconfiguring its aldehyde group for their own specialized needs.
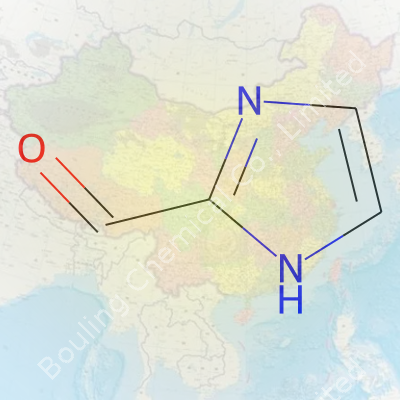
Anybody who’s spent time peering under the hood of a molecule has run across imidazole at some point. This little ring shows up all over nature—think histidine, the amino acid every biochemist sweats over. Now, take imidazole, stick a formyl group (-CHO) at the carbon next to one of the nitrogens, and you’re staring at 2-Formylimidazole.
Let’s lay it out: 2-Formylimidazole’s molecular formula is C4H4N2O. It packs four carbons, four hydrogens, two nitrogens, and one oxygen into a five-membered ring with a touch of character at the second spot.
Chemists rarely look at a formula as just a set of letters and numbers. Structure tells the whole story. You’ve got a five-membered imidazole ring: two of those ring atoms are nitrogens at the 1- and 3-positions. The formyl group slings onto the 2-position—right beside the N1. That formyl carbon links via a double bond to an oxygen atom, giving us a classic aldehyde group staring out from the ring.
Drawing it out, the imidazole has those alternating double bonds making it aromatic and planar. This shape holds together thanks to resonance. Aromaticity means the electrons zip around the ring in a way that makes it stable—always a plus for reactivity. Attach that formyl at the 2-position, and now the ring shifts to play with nucleophiles or slip into more complex syntheses.
This is no lab curiosity—it’s got bite. In many fields, 2-Formylimidazole pops up as a key intermediate. For instance, it slips into pharmaceutical research, helps design new compounds, and even serves as a building block for heterocyclic libraries. I remember long days in the lab, looking for starting materials that could keep imidazole’s stability but still offer a reactive handle. The formyl group does just that, letting chemists bolt on new structures, build libraries, or tweak properties to chase after new drug candidates.
It shows up in the real world too. Folks in environmental chemistry analyze it in food and beverage systems, especially after the Maillard reaction runs its course during roasting or caramelization. That changes flavor and color, but sometimes, these compounds build up more than you’d expect.
Having an aldehyde group in a heterocycle isn’t always a walk in the park. Aldehydes like to react, which can spell trouble for storage or lead to unexpected byproducts in synthesis. In the lab, I’ve seen 2-Formylimidazole pick up moisture or react with other nearby amines if you leave it exposed to air for too long. That means you have to keep it pretty dry and use it fresh in reactions. Wrangling purity can take some effort—column chromatography, followed by careful NMR checks.
On the environmental side, knowing how and where 2-Formylimidazole forms lets us monitor food safety. As people pay more attention to how their food is processed, tracking small molecules like this one becomes important. Analytical methods like high-performance liquid chromatography (HPLC) keep getting better, helping chemists at food companies keep tabs on flavor profiles and potential toxins.
2-Formylimidazole stands out for its marriage of stability and reactivity. The simple step of dropping a formyl group onto imidazole turns the molecule into a valuable crossroads in the lab and in daily life. Understanding both its formula and structure pays off every time a chemist plots a new synthesis or analyzes what’s really brewing inside a roasted coffee bean.
Walk into any lab where scientists spend their time making medicines, and you’ll probably find 2-Formylimidazole tucked somewhere on a shelf. The name sounds technical, but the reason this compound crops up so often connects with the way it acts almost like a building block. Anyone who’s taken some chemistry remembers the challenge of linking together molecules in useful ways. That push and pull to find ingredients that actually work together comes up every day for chemists building pharmaceuticals.
People always want faster painkillers, better antibiotics, and fewer side effects. Drug developers reach for 2-Formylimidazole since it reacts well with so many chemicals. They use it to set up the base for more involved molecules. It’s not flashy by itself. The real draw comes from the chance to snap on other groups and turn it into something active in the body. Big names in pharma rely on this approach to churn out batches of experimental drugs without wasting weeks tweaking recipes no one will use again.
I’ve seen colleagues in research labs search for options that won’t clog up during a reaction or break apart at the wrong time. 2-Formylimidazole steps in, offering a solid base that behaves reliably whether mixed with other small molecules or more complicated agents. The consistency makes scientists’ days easier. Rather than dreading what could go wrong, they can focus on what molecules might cure the next round of tough diseases.
Testing chemicals sometimes sounds simple, but every chemist recognizes the grind of running controls and checking what comes out the other side. Analytical chemistry teams use 2-Formylimidazole to trap other chemicals and help spot contamination. Its “stickiness” to certain metals or stray bits in mixtures makes cleanup quicker. That’s not just a time-saver — reducing mess lowers cost and limits exposure to risky materials.
In factories, quality checks keep lines moving. Additives sometimes sneak into the mix from leftover metals or other substances used earlier in the process. Here, 2-Formylimidazole tags those hidden extras, making them easier to pull out and measure. In my experience, watching a plant manager breathe easier because of fewer shutdowns shows the real benefit. Less downtime means people keep working and products reach their destination faster.
Modern gadgets keep shrinking, and circuitry grows more complicated every year. The electronics industry grabs chemicals like 2-Formylimidazole for tweaking how metals deposit on chips. It works as a ligand — a word for something that grabs a metal atom and hangs on during delicate reactions — and helps create thin, even layers. That precision determines not just how fast a chip runs, but also its lifespan. Using trusted intermediates cuts down on failed batches and wasted material.
Lots of specialty chemicals fade out over time as trends shift, but this one carries on because it never tries to do all the work. Giving chemists, engineers, and analysts the right tools helps them solve bigger problems — whether in making new medicine, keeping production lines safe, or building tomorrow’s electronics. That’s what keeps demand going.
Chemistry labs have always made me think twice about tiny bottles lined up on shelves. One name that pops up is 2-Formylimidazole. It’s a common pick for researchers working in pharmaceuticals, fine chemicals, and a bunch of tricky synthesis tasks. What stands out about this compound isn’t its label, but just how quirky it acts if you give it half a chance. Left unchecked, its quality can plummet fast.
Leave 2-Formylimidazole in the wrong spot, and you’re playing with fire. It reacts to air, light, moisture—like a toddler let loose in a candy shop. Over the years, I’ve seen careless storage turn this solid yellow powder into a mess of by-products and guesswork. Exposure to humidity leads to hydrolysis. Light means slow but steady decomposition. Even oxygen will chip away at its integrity over time.
A colleague once tucked a bottle in a bin, lid a little loose. Not long after, moldy smells and dark spots greeted anyone poking around. That sample landed in the hazardous waste, costing both time and money.
Store 2-Formylimidazole like you’re saving a family recipe for the next generation. Air-tight containers matter. Glass bottles with solid screw tops work best. Anything leaky turns a week’s supply into trash. Keep it cool and dark. A cabinet between 2-8°C, like those found in standard lab refrigerators, easily extends shelf life. I’ve learned not to let the container warm up even briefly during handling—temperature swings invite condensation inside.
For those who want numbers: studies report samples hold their punch for over a year under these chill, dry, and dark conditions. Let the room get warm and damp, though, and color changes—or, worse, impurity formation—kick in within months. Some folks use desiccators to take the moisture down a notch further, especially in muggy climates.
I used to think a label with a date would do the job. Turns out, routine checks make a bigger difference. Any sign of clumping, color shifts, or weird odors call for a new batch. Labs with strict hygiene standards set up regular inventory audits, tossing anything expired or showing signs of breakdown. It saves everyone from ruined experiments and even potential safety issues.
The best labs also keep a written log—every time a bottle gets opened or moves spots, someone marks it down. That way, there’s no guessing about where it’s been or what conditions it went through.
A handful of habits protect against waste. Buy only what you’ll use in a reasonable time. Split larger purchases into smaller, well-labeled containers. For short-term access, some even wrap a portion in foil to block light while working at the bench. Avoid mixing old and new material. These small steps mean less material wasted and cleaner, more reliable results.
Dealing with chemicals like 2-Formylimidazole teaches respect for routines. Good storage isn’t just about following a rulebook. It’s based on mistakes and fixes—real learning that sticks better than any printed sheet. Keeping this compound stable becomes more than just precaution—it’s about protecting work, budgets, and health. That lesson stays with me every time I step into a lab.
2-Formylimidazole doesn’t turn up on mainstream news feeds, but folks in labs and chemical plants know it’s a reality for resin manufacturing, drugs, and a few niche chemical syntheses. Its formula looks simple enough, yet the moment someone mentions “safety,” opinions start piling up. Let’s cut through the confusion and talk straight about what to look out for and why respect for this chemical matters.
Every chemical has a personality, and 2-Formylimidazole likes to keep people on their toes. It doesn’t explode without warning, but its low vapor pressure doesn’t mean you should breathe it in. Inhaling particles or dust during routine weighing or measurement can lead to irritation in the nose or throat. Getting some on the skin? Expect a possible rash or redness, not the end of the world, but enough to make you wish you’d worn gloves.
Spilling some on a sleeve won’t send you to the emergency room, but the way it dries on skin seems to stretch out the discomfort. Eyes get watery and irritated quickly if dust floats up. I’ve seen someone rub their eye in the middle of a synthesis and regret it instantly.
Some folks spend years working with aldehydes and think a dash of chemical risk comes with the territory. That kind of thinking invites trouble. Animal tests don’t always translate directly to people, but 2-Formylimidazole’s chemical cousins sometimes trigger longer-term concerns, including potential cancer hazards. The Material Safety Data Sheet doesn’t have “hazardous” in big, bold letters, but reading between the lines makes me treat it as more than a minor nuisance.
Mild or not, lab culture doesn't reward bravado. Consistent glove use, a fume hood, and protective eyewear turn a tricky job into an ordinary one. Regular hand-washing and changing out gloves every time a new batch starts take only a few extra seconds, and that’s sometimes all it takes to avoid months of repeated skin dryness.
Back when I did daily syntheses, I noticed the biggest hassle came from working in cramped spaces. Any crowded lab creates more spills and more nagging about personal protective gear. Setting up spray bottles of isopropyl alcohol near benches helps, especially when dealing with aldehydes that cling to glassware and gloves. For small-scale measuring, weighing paper folded into troughs saved more time than trying to sweep up stray powder later.
If a lab can invest in powder-tight vials or use pre-dispensed capsules, the benefits roll in quickly. Automation sometimes earns its keep just by limiting the moments when people handle a powder directly. Not every workplace can afford a robot or sealed system, but the tiniest tweaks to storage — think snap jars or sealed bags — keep the headaches to a minimum.
Writing more hazard labels in big letters wouldn’t do as much as a manager who celebrates quick clean-ups and checks on broken gloves. Respecting the chemical and its risks isn’t about working with fear; it’s about recognizing limits, sharing what goes wrong, and chipping away at complacency. People stay safer when routines matter more than shortcuts, especially around chemicals that only reveal their bite after repeated carelessness.
In the world of specialty chemicals, picking the right grade and package size isn't a trivial decision—it can mean the difference between a project running smoothly or a lab grinding to a halt. Take 2-Formylimidazole, a compound with its feet in pharmaceuticals, agrochemicals, and chemical synthesis. The question of which purity grade and packaging size makes sense isn’t just paperwork for the purchasing department. It rolls right down to the bench, where every contaminant or shortfall can spark problems that ripple through the entire workflow.
Anyone who's worked in a research lab knows that not all chemicals are created equal, even when they share the same name. Most 2-Formylimidazole on the market clocks in at about 97% to 99% purity. This isn't arbitrary. In pharmaceutical research, even a one percent impurity can gum up an entire synthesis or foul up bioactivity testing. High purity—typically at least 98%—helps avoid headaches far down the pipeline, from chromatographic separations to reaction yields.
Technical grade hovers at about 97% purity, which usually works for early-stage discovery or less critical applications. When projects move into regulatory territory or analytical chemistry, scientists reach for 98% or higher, often labeled as reagent or analytical grade. In my own experience, the few times I gambled on lower-grade material, calibration curves fell apart, and I spent more time troubleshooting than doing actual chemistry. These numbers on a datasheet aren't empty boasts, they're the handrails that keep a project on track.
Small labs, contract research organizations, and manufacturing outfits all see packaging from different vantage points. Glass bottles and HDPE containers dominate, usually in 5g, 25g, 100g, or 500g sizes. Those developing new chemical methods might only need 5 or 10 grams. I've been there—ordering a tiny amber vial and wondering if it’ll even last a month of experiments. For scale-up or pilot plant work, 500g drums hit the sweet spot, enough to fuel several runs without exposing too much product to moisture or air.
Too large a package, and the chemical risks absorbing water from the air each time it’s opened, which can turn something as innocuous as opening a bottle into an expensive mistake. Too small, and every experiment feels like rummaging in an empty cupboard, rationing every milligram. I once spent half a morning scraping the bottom of a container, wishing I’d thought further ahead about how much material the project would really chew through.
Some chemists chase the highest purity out of habit, but cost weighs heavy. Analytical grade chemicals hit budgets much harder than technical grade, and for a big team, those price differences can add up quickly. It's tempting to save a few bucks up front, but downstream surprises almost always wind up costing more. I've watched reactions stall out because metal ions from a “cheap” batch tainted sensitive steps. On the flip side, ordering excess high-purity product, then watching it degrade on the back shelf, feels just as short-sighted.
Suppliers usually step up with safety data sheets and certificates of analysis, which help nail down exactly what’s inside each bottle. Still, nothing beats a quick internal check—running a control reaction or checking NMR for odd peaks—before betting the project on a fresh shipment.
There’s no single “best” choice for everyone. Picking the right grade and package size means weighing purity, budget, frequency of use, and shelf life—all at once. For anyone handling 2-Formylimidazole, it's worth remembering that chemicals have a way of exposing shortcuts in planning. Taking the time to get informed pays off, not just in data quality but in fewer headaches down the road.
| Names | |
| Preferred IUPAC name | 1H-imidazole-2-carbaldehyde |
| Other names |
Glyoxal monoxime 2-Imidazolecarboxaldehyde Imidazole-2-carbaldehyde Imidazole-2-aldehyde |
| Pronunciation | /tuː-ˈfɔːrmɪl-ɪˈmɪdəˌzɔːl/ |
| Identifiers | |
| CAS Number | 670-50-4 |
| Beilstein Reference | 120793 |
| ChEBI | CHEBI:73038 |
| ChEMBL | CHEMBL1231167 |
| ChemSpider | 154922 |
| DrugBank | DB04162 |
| ECHA InfoCard | 100.021.627 |
| EC Number | 674-36-0 |
| Gmelin Reference | 780132 |
| KEGG | C11933 |
| MeSH | D000080364 |
| PubChem CID | 69706 |
| RTECS number | NR3500000 |
| UNII | N4L6J4C26P |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C4H4N2O |
| Molar mass | 110.10 g/mol |
| Appearance | White to pale yellow crystalline powder |
| Odor | odourless |
| Density | 1.21 g/cm3 |
| Solubility in water | soluble |
| log P | 0.02 |
| Vapor pressure | 0.00182 mmHg at 25°C |
| Acidity (pKa) | 7.0 |
| Basicity (pKb) | 11.10 |
| Magnetic susceptibility (χ) | -62.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.632 |
| Dipole moment | 1.98 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 154.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -59.3 kJ mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -5076.6 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P280, P261, P304+P340, P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | > 167°C |
| Autoignition temperature | 250 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat) > 4200 mg/kg |
| LD50 (median dose) | LD50 (median dose): 372 mg/kg (oral, rat) |
| PEL (Permissible) | Not established |
| REL (Recommended) | REL: NIOSH considers 2-formylimidazole to be a potential occupational carcinogen; no REL established |
| Related compounds | |
| Related compounds |
Imidazole 4-Formylimidazole 2-Methylimidazole 2-Nitroimidazole 2-Acetylimidazole |